Abstract
Aim—To evaluate immunophenotyping by means of flow cytometry as a complementary method for the detection of malignant cells in serous effusions and peritoneal washings.
Material and methods—Frozen samples of 49 fresh serous effusions and peritoneal washings were analysed by flow cytometry, using monoclonal antibodies against CD45, Ber-EP4, and N-cadherin. Results were compared with smear and cell block morphology, as well as immunocytochemistry on paraffin wax embedded cell blocks.
Results—Seventeen specimens were cytologically diagnosed as malignant, whereas 25 were interpreted as benign. The remaining seven specimens were diagnosed as indeterminate or suspicious for malignancy. Ber-EP4 positive cells were detected in 16 of the 17 cytologically malignant effusions, as well as in five of seven suspicious cases and five of 25 specimens with benign cytology. In the latter group, three specimens showed atypical or malignant cell groups that were missed in routine morphological evaluation. In two additional samples, obtained from patients with benign and borderline ovarian tumours, Ber-EP4 positive cells showed benign or mildly atypical features, and were interpreted as exfoliated benign or borderline malignant epithelial cells of tubal origin, or as endosalpingiosis. All five Ber-EP4 positive indeterminate specimens showed atypical or malignant cells on re-evaluation, and were Ber-EP4 positive in four of five cases using immunohistochemistry in cell block sections. Large numbers of CD45 positive and relatively few N-cadherin positive cells were detected in most specimens with the use of flow cytometry, when compared with morphological evaluation.
Conclusions—Flow cytometry is a rapid and highly effective method for the evaluation of effusions and peritoneal washings. The detection of Ber-EP4 positive cells using flow cytometry is strongly indicative of the presence of carcinoma cells in effusions and peritoneal washings. Although false positives are relatively infrequent, all specimens should be carefully evaluated morphologically to prevent the diagnosis of benign epithelial clusters as malignant.
Key Words: flow cytometry • serous effusions • immunophenotyping • cytology • Ber-EP4 • N-cadherin
Full Text
The Full Text of this article is available as a PDF (138.2 KB).
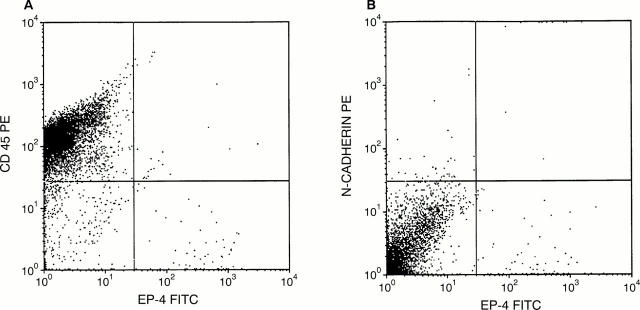
Figure 1 Flow cytometry results of a metastatic carcinoma of unknown origin, showing unequivocal Ber-EP4 positive cells, as well as large numbers of CD45 positive cells and a few N-cadherin positive cells.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedrossian C. W. Diagnostic problems in serous effusions. Diagn Cytopathol. 1998 Aug;19(2):131–137. doi: 10.1002/(sici)1097-0339(199808)19:2<131::aid-dc14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ceyhan B. B., Demiralp E., Celikel T. Analysis of pleural effusions using flow cytometry. Respiration. 1996;63(1):17–24. doi: 10.1159/000196510. [DOI] [PubMed] [Google Scholar]
- Chen L. M., Lazcano O., Katzmann J. A., Kimlinger T. K., Li C. Y. The role of conventional cytology, immunocytochemistry, and flow cytometric DNA ploidy in the evaluation of body cavity fluids: a prospective study of 52 patients. Am J Clin Pathol. 1998 Jun;109(6):712–721. doi: 10.1093/ajcp/109.6.712. [DOI] [PubMed] [Google Scholar]
- Czerniak B., Papenhausen P. R., Herz F., Koss L. G. Flow cytometric identification of cancer cells in effusions with Ca1 monoclonal antibody. Cancer. 1985 Jun 15;55(12):2783–2788. doi: 10.1002/1097-0142(19850615)55:12<2783::aid-cncr2820551211>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Davidson B., Risberg B., Kristensen G., Kvalheim G., Emilsen E., Bjåmer A., Berner A. Detection of cancer cells in effusions from patients diagnosed with gynaecological malignancies. Evaluation of five epithelial markers. Virchows Arch. 1999 Jul;435(1):43–49. doi: 10.1007/s004280050393. [DOI] [PubMed] [Google Scholar]
- Diaz-Arias A. A., Loy T. S., Bickel J. T., Chapman R. K. Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol. 1993 Oct;9(5):516–521. doi: 10.1002/dc.2840090509. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Thornthwaite J. T., Ng A. B., Sugarbaker E. V. DNA flow cytometry of pleural effusions. Comparison with pathology for the diagnosis of malignancy. Anal Quant Cytol. 1983 Mar;5(1):19–27. [PubMed] [Google Scholar]
- Flynn M. K., Johnston W., Bigner S. Carcinoma of ovarian and other origins in effusions. Immunocytochemical study with a panel of monoclonal antibodies. Acta Cytol. 1993 Jul-Aug;37(4):439–447. [PubMed] [Google Scholar]
- Geiger B., Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Green L. K., Griffin J. Increased natural killer cells in fluids. A new, sensitive means of detecting carcinoma. Acta Cytol. 1996 Nov-Dec;40(6):1240–1245. doi: 10.1159/000333987. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Han A. C., Filstein M. R., Hunt J. V., Soler A. P., Knudsen K. A., Salazar H. N-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas: a ThinPrep immunocytochemical study. Cancer. 1999 Apr 25;87(2):83–86. doi: 10.1002/(sici)1097-0142(19990425)87:2<83::aid-cncr7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Han A. C., Peralta-Soler A., Knudsen K. A., Wheelock M. J., Johnson K. R., Salazar H. Differential expression of N-cadherin in pleural mesotheliomas and E-cadherin in lung adenocarcinomas in formalin-fixed, paraffin-embedded tissues. Hum Pathol. 1997 Jun;28(6):641–645. doi: 10.1016/s0046-8177(97)90171-4. [DOI] [PubMed] [Google Scholar]
- Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987 Mar;120(1):215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Joseph M. G., Banerjee D., Harris P., Gibson S., McFadden R. G. Multiparameter flow cytometric DNA analysis of effusions: a prospective study of 36 cases compared with routine cytology and immunohistochemistry. Mod Pathol. 1995 Aug;8(6):686–693. [PubMed] [Google Scholar]
- Knudsen K. A., Myers L., McElwee S. A. A role for the Ca2(+)-dependent adhesion molecule, N-cadherin, in myoblast interaction during myogenesis. Exp Cell Res. 1990 Jun;188(2):175–184. doi: 10.1016/0014-4827(90)90157-6. [DOI] [PubMed] [Google Scholar]
- Latza U., Niedobitek G., Schwarting R., Nekarda H., Stein H. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol. 1990 Mar;43(3):213–219. doi: 10.1136/jcp.43.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordóez N. G. Value of the Ber-EP4 antibody in differentiating epithelial pleural mesothelioma from adenocarcinoma. The M.D. Anderson experience and a critical review of the literature. Am J Clin Pathol. 1998 Jan;109(1):85–89. doi: 10.1093/ajcp/109.1.85. [DOI] [PubMed] [Google Scholar]
- Peralta Soler A., Knudsen K. A., Jaurand M. C., Johnson K. R., Wheelock M. J., Klein-Szanto A. J., Salazar H. The differential expression of N-cadherin and E-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas. Hum Pathol. 1995 Dec;26(12):1363–1369. doi: 10.1016/0046-8177(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Redies C., Engelhart K., Takeichi M. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J Comp Neurol. 1993 Jul 15;333(3):398–416. doi: 10.1002/cne.903330307. [DOI] [PubMed] [Google Scholar]
- Rijken A., Dekker A., Taylor S., Hoffman P., Blank M., Krause J. R. Diagnostic value of DNA analysis in effusions by flow cytometry and image analysis. A prospective study on 102 patients as compared with cytologic examination. Am J Clin Pathol. 1991 Jan;95(1):6–12. doi: 10.1093/ajcp/95.1.6. [DOI] [PubMed] [Google Scholar]
- Robinson R. J., Royston D. Comparison of monoclonal antibodies AUA1 and BER EP4 with anti-CEA for detecting carcinoma cells in serous effusions and distinguishing them from mesothelial cells. Cytopathology. 1993;4(5):267–271. doi: 10.1111/j.1365-2303.1993.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Sabile A., Louha M., Bonte E., Poussin K., Vona G., Mejean A., Chretien Y., Bougas L., Lacour B., Capron F. Efficiency of Ber-EP4 antibody for isolating circulating epithelial tumor cells before RT-PCR detection. Am J Clin Pathol. 1999 Aug;112(2):171–178. doi: 10.1093/ajcp/112.2.171. [DOI] [PubMed] [Google Scholar]
- Schneller J., Eppich E., Greenebaum E., Elequin F., Sherman A., Wersto R., Koss L. G. Flow cytometry and Feulgen cytophotometry in evaluation of effusions. Cancer. 1987 Apr 1;59(7):1307–1313. doi: 10.1002/1097-0142(19870401)59:7<1307::aid-cncr2820590713>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sikora J., Dworacki G., Trybus M., Batura-Gabryel H., Zeromski J. Correlation between DNA content, expression of Ki-67 antigen of tumor cells and immunophenotype of lymphocytes from malignant pleural effusions. Tumour Biol. 1998;19(3):196–204. doi: 10.1159/000030007. [DOI] [PubMed] [Google Scholar]
- Sikora J., Dworacki G., Zeromski J. DNA ploidy, S-phase, and Ki-67 antigen expression in the evaluation of cell content of pleural effusions. Lung. 1996;174(5):303–313. doi: 10.1007/BF00176189. [DOI] [PubMed] [Google Scholar]
- Simsir A., Fetsch P., Mehta D., Zakowski M., Abati A. E-cadherin, N-cadherin, and calretinin in pleural effusions: the good, the bad, the worthless. Diagn Cytopathol. 1999 Mar;20(3):125–130. doi: 10.1002/(sici)1097-0339(199903)20:3<125::aid-dc3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Soler A. P., Knudsen K. A. N-cadherin involvement in cardiac myocyte interaction and myofibrillogenesis. Dev Biol. 1994 Mar;162(1):9–17. doi: 10.1006/dbio.1994.1062. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tamai M., Tanimura H., Yamaue H., Iwahashi M., Tsunoda T., Tani M., Noguchi K., Hotta T., Arii K. Expression of carcinoembryonic antigen in fresh human gastric cancer cells assessed by flow cytometry. J Surg Oncol. 1993 Mar;52(3):176–180. doi: 10.1002/jso.2930520312. [DOI] [PubMed] [Google Scholar]
- Unger K. M., Raber M., Bedrossian C. W., Stein D. A., Barlogie B. Analysis of pleural effusions using automated flow cytometry. Cancer. 1983 Sep 1;52(5):873–877. doi: 10.1002/1097-0142(19830901)52:5<873::aid-cncr2820520522>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]