Abstract
Aims—A routine immunohistochemical (IHC) assay is now commonly used for determining the oestrogen receptor (ER) and progesterone receptor (PR) status of women with breast cancer. To date, no large studies have been conducted that report the expected frequency of receptor positivity in relation to patient age and sensitivity of the IHC assay. Data on 7016 breast carcinomas from 71 laboratories were analysed to determine the frequency of receptor positivity and investigate possible causes of the observed variation in detection rates.
Methods—Members of UK NEQAS-ICC (UK National External Quality Assessment Scheme for Immunocytochemistry) provided data on the receptor status of cases routinely assayed in their departments over a period of two to 26 months between June 1996 and September 1998. Data on 7016 breast carcinomas were stratified according to patient age and receptor status. Frequency of receptor positivity was correlated with IHC assay sensitivity, the threshold value used to determine receptor positivity, and the presence or absence of mammographic screening in the hospitals or clinics served by the laboratories.
Results—The highest proportion of receptor positive cases occurred in patients in the age ranges > 65 years for ER and 41–50 years for PR. There was a significant positive correlation between frequency of receptor positivity and the sensitivity of the IHC assay, for both ER (rs = 0.346; p = 0.019; two tailed) and PR (rs = 0.561; p = 0.003; two tailed). The mean frequency of receptor positivity for laboratories using the same 10% threshold value was 77% for ER (95% confidence interval (CI), 74% to 80%) in laboratories with high sensitivity and 72% (95% CI, 68% to 76%) for those with low assay sensitivity (p = 0.025). For PR, the mean frequency of receptor positivity for laboratories using the same 10% threshold value and having high assay sensitivity was 63% (95% CI, 57% to 69%), and 51% (95% CI, 38% to 65%) for laboratories with assays of low sensitivity (p = 0.022). The mean frequency of ER positivity for laboratories serving hospitals and clinics where mammographic screening does and does not take place was 73.4% and 75.7%, respectively (p = 0.302; two tailed).
Conclusions—Of the parameters investigated, patient age and IHC assay sensitivity were found to be the main variables influencing the frequency of receptor positivity. We recommend the range of receptor values obtained by laboratories achieving high assay sensitivity as a useful guide against which all laboratories can gauge their own results.
Key Words: immunohistochemistry • oestrogen receptors • progesterone receptors • frequency of positivity
Full Text
The Full Text of this article is available as a PDF (166.7 KB).
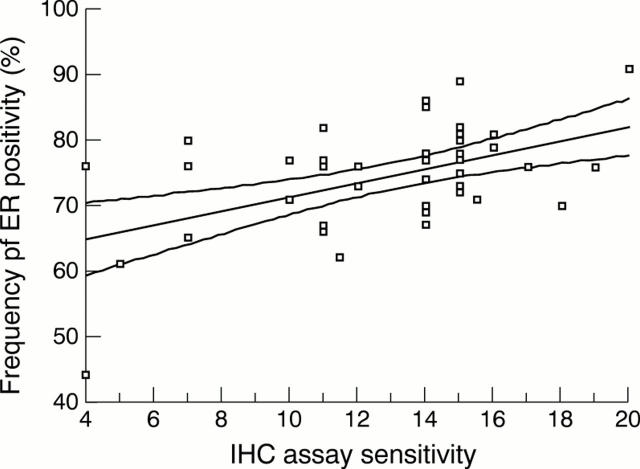
Figure 1 Scatter diagram to show the relation between the frequency of oestrogen receptor (ER) positivity in 46 laboratories and the immunohistochemical (ICH) assay sensitivity (median score achieved for assessment runs 40, 41, and 42). A least squares linear regression line is shown with 95% confidence interval, giving the best fit for all the data points. Spearman's correlation coefficient = 0.346, p = 0.019 (two tailed).
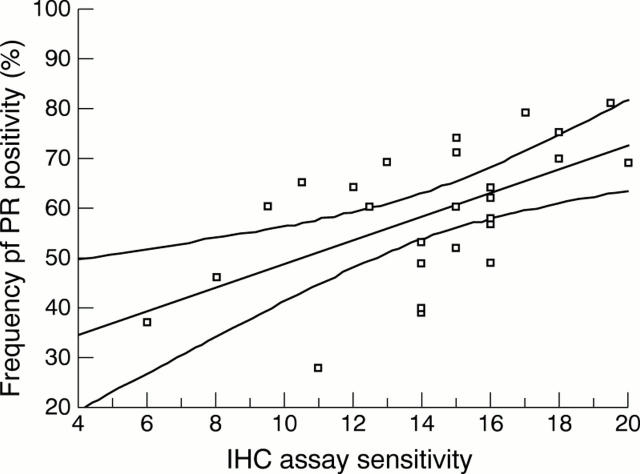
Figure 2 Scatter diagram to show the relation between the frequency of progesterone receptor (PR) positivity in 46 laboratories and the immunohistochemical (ICH) assay sensitivity (median score achieved for assessment runs 36, 39, and 41). A least squares linear regression line is shown with 95% confidence interval, giving the best fit for all the data points. Spearman's correlation coefficient = 0.561, p = 0.003 (two tailed).
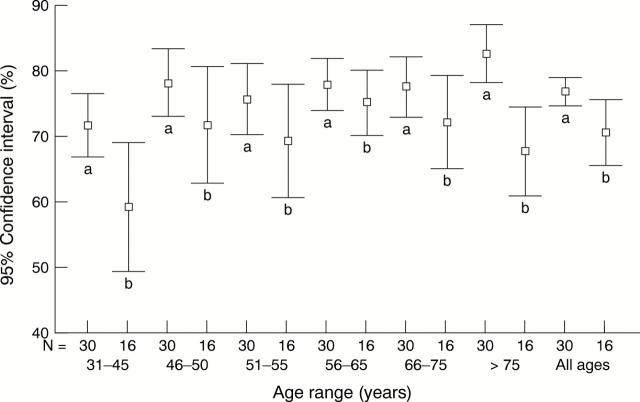
Figure 3 The mean frequency of oestrogen receptor (ER) positivity at different patient age ranges for laboratories with high assay sensitivity (n = 30, labelled "a") and low assay sensitivity (n = 16, labelled "b"). High assay sensitivity is defined as a median score ≥ 13/20 in assessment runs 40, 41, and 42; low assay sensitivity is defined as a median score ≤12/20 in assessment runs 40, 41, and 42.
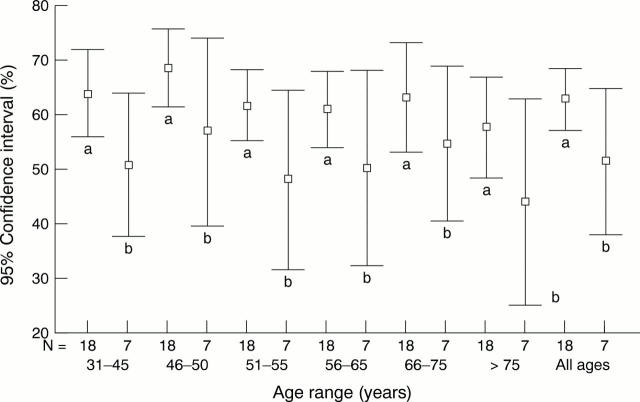
Figure 4 The mean frequency of progesterone receptor (PR) positivity at different patient age ranges for laboratories with high assay sensitivity (n = 18, labelled "a") and low assay sensitivity (n = 7, labelled "b"). High assay sensitivity is defined as a median score ≥ 13/20 in assessment runs 36, 39, and 41; low assay sensitivity is defined as a median score ≤12/20 in assessment runs 36, 39, and 41.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Bustamante M. A., Daniel C. O., Gaskill H. V., Cruz A. B., Jr Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990 Jan;125(1):107–113. doi: 10.1001/archsurg.1990.01410130113018. [DOI] [PubMed] [Google Scholar]
- Allred D. C., Harvey J. M., Berardo M., Clark G. M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998 Feb;11(2):155–168. [PubMed] [Google Scholar]
- Barnes D. M., Harris W. H., Smith P., Millis R. R., Rubens R. D. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996 Nov;74(9):1445–1451. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. M., Millis R. R., Beex L. V., Thorpe S. M., Leake R. E. Increased use of immunohistochemistry for oestrogen receptor measurement in mammary carcinoma: the need for quality assurance. Eur J Cancer. 1998 Oct;34(11):1677–1682. doi: 10.1016/s0959-8049(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Elledge R. M., Osborne C. K. Oestrogen receptors and breast cancer. BMJ. 1997 Jun 28;314(7098):1843–1844. doi: 10.1136/bmj.314.7098.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernö M., Andersson C., Fallenius G., Idvall I. Oestrogen receptor analysis of paraffin sections and cytosol samples of primary breast cancer in relation to outcome after adjuvant tamoxifen treatment. The South Sweden Breast Cancer Group. Acta Oncol. 1996;35(1):17–22. doi: 10.3109/02841869609098474. [DOI] [PubMed] [Google Scholar]
- Harvey J. M., Clark G. M., Osborne C. K., Allred D. C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999 May;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- Hendricks J. B., Wilkinson E. J. Comparison of two antibodies for evaluation of estrogen receptors in paraffin-embedded tumors. Mod Pathol. 1993 Nov;6(6):765–770. [PubMed] [Google Scholar]
- Howanitz P. J. Quality assurance measurements in departments of pathology and laboratory medicine. Arch Pathol Lab Med. 1990 Nov;114(11):1131–1135. [PubMed] [Google Scholar]
- McGuire W. L., Chamness G. C., Fuqua S. A. Estrogen receptor variants in clinical breast cancer. Mol Endocrinol. 1991 Nov;5(11):1571–1577. doi: 10.1210/mend-5-11-1571. [DOI] [PubMed] [Google Scholar]
- Pertschuk L. P., Feldman J. G., Kim Y. D., Braithwaite L., Schneider F., Braverman A. S., Axiotis C. Estrogen receptor immunocytochemistry in paraffin embedded tissues with ER1D5 predicts breast cancer endocrine response more accurately than H222Sp gamma in frozen sections or cytosol-based ligand-binding assays. Cancer. 1996 Jun 15;77(12):2514–2519. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2514::AID-CNCR14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Reiner A., Neumeister B., Spona J., Reiner G., Schemper M., Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990 Nov 1;50(21):7057–7061. [PubMed] [Google Scholar]
- Rhodes A., Jasani B., Balaton A. J., Miller K. D. Immunohistochemical demonstration of oestrogen and progesterone receptors: correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries. J Clin Pathol. 2000 Apr;53(4):292–301. doi: 10.1136/jcp.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes A., Jasani B., Barnes D. M., Bobrow L. G., Miller K. D. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000 Feb;53(2):125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain S., Lainé Bidron C., Martin P. M., Magdelenat H. Steroid receptor distribution in 47,892 breast cancers. A collaborative study of 7 European laboratories. The EORTC Receptor Study Group. Eur J Cancer. 1995;31A(3):411–417. doi: 10.1016/0959-8049(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Saccani Jotti G., Johnston S. R., Salter J., Detre S., Dowsett M. Comparison of new immunohistochemical assay for oestrogen receptor in paraffin wax embedded breast carcinoma tissue with quantitative enzyme immunoassay. J Clin Pathol. 1994 Oct;47(10):900–905. doi: 10.1136/jcp.47.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran I., Quénel N., Coindre J. M., Bonichon F., Durand M., Wafflart J., Mauriac L. pS2 protein: a marker improving prediction of response to neoadjuvant tamoxifen in post-menopausal breast cancer patients. Br J Cancer. 1996 Oct;74(7):1120–1125. doi: 10.1038/bjc.1996.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Mepham B. L., Wright D. H. Tissue preparation for immunocytochemistry. J Clin Pathol. 1997 May;50(5):422–428. doi: 10.1136/jcp.50.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Saati T., Clamens S., Cohen-Knafo E., Faye J. C., Prats H., Coindre J. M., Wafflart J., Caverivière P., Bayard F., Delsol G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer. 1993 Oct 21;55(4):651–654. doi: 10.1002/ijc.2910550423. [DOI] [PubMed] [Google Scholar]
- van Diest P. J., van Dam P., Henzen-Logmans S. C., Berns E., van der Burg M. E., Green J., Vergote I. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997 Oct;50(10):801–804. doi: 10.1136/jcp.50.10.801. [DOI] [PMC free article] [PubMed] [Google Scholar]