Abstract
Aims—To investigate whether increasing the daily baseline of gut calcium can cause a gradual downregulation of the active intestinal transport of calcium via reduced parathyroid hormone (PTH) mediated activation of vitamin D, and to discuss why such a mechanism might prevent calcium oxalate rich stones. To demonstrate the importance of seasonal effects upon the evaluation of such data.
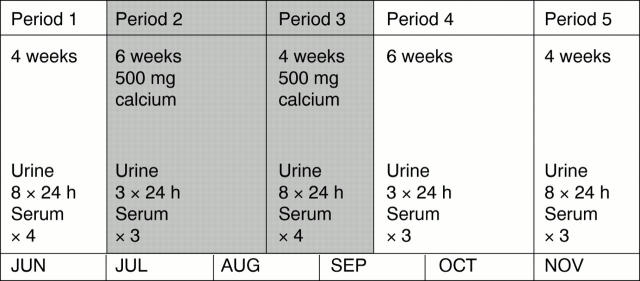
Methods—Within an intensive 24 hour urine collection regimen, daily calcium supplementation (500 mg) was given to five stone formers for a 10 week period during a six month crossover study. In a further population of patients on follow up for previous renal stone disease, observations were made on 1066 24 hour urine samples collected over five years in respect of seasonal effects relevant to the interpretation of the study.
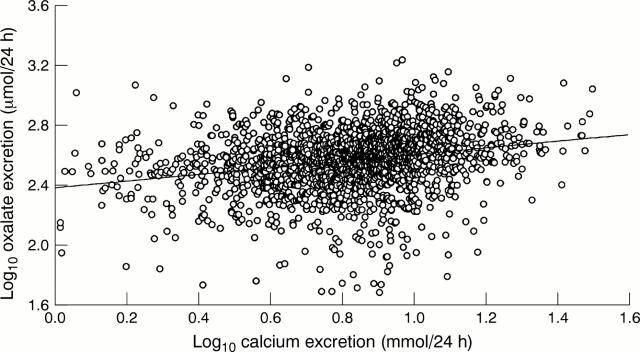
Results—In the group of patients on calcium supplements the following results were found. During calcium supplementation, the proportion of urine calcium to oxalate was higher (increased calcium to oxalate molar ratio), the 24 hour urine product of calcium and oxalate did not rise, and urine oxalate was lower during the first six weeks of supplementation. Twenty four hour urine calcium was 10.2% higher than baseline in the final four weeks of the 10 weeks of supplementation. Twenty four hour urine phosphate was 11.4% lower during the first six weeks of supplementation, but then rose while the patients were still on supplementation; renal tubular reabsorption of phosphate (TmP/GFR) mirrored the urine phosphate changes inversely. PTH was higher after stopping supplementation, but 1,25-(OH)2-cholecalciferol changes were not detected. In the 1066 urine samples collected over five years the following results were found. Calcium and oxalate excretion correlated positively and not inversely. Urine calcium and phosphate excretion were 5.5% and 2.5% higher, respectively, in "light" months of the year compared with "dark" months. A post summer decline in both urine calcium and urine phosphate was relevant to the interpretation of the study.
Conclusions—Regular calcium supplementation does not raise the product of calcium and oxalate in urine and the proportion of oxalate to calcium is reduced. The underlying mechanisms of the changes seen in phosphate, calcium, and PTH and the observations on 1,25-(OH)2-cholecalciferol are not clear. Observed changes in phosphate could possibly be part of a calcium regulating feedback loop operating over a period of weeks. In evaluating these mechanisms background seasonal effects are important. It is possible that "programming" of the gut mucosa in terms of calcium transport is a major determinant of the relation between calcium and oxalate concentrations in urine and their relative abundance. Increased oral calcium, in association with a reduction of the relative proportion absorbed, may be pertinent to the prevention of calcium oxalate rich stones.
Key Words: renal stones • calcium oxalate product • dietary calcium • renal tubular reabsorption of phosphate
Full Text
The Full Text of this article is available as a PDF (202.6 KB).
Figure 1 Illustration of the trial design showing the periods when calcium supplements were given and the timing of blood and urine samples.
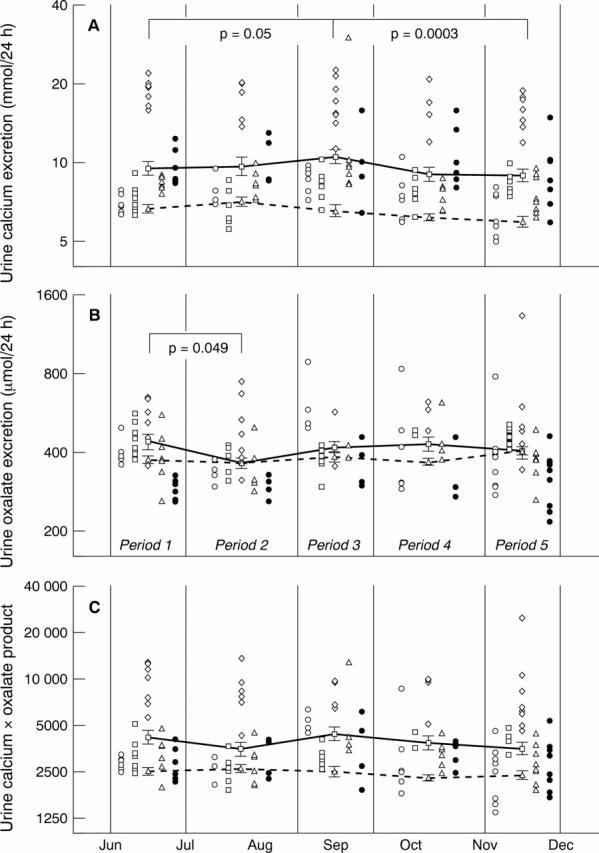
Figure 2 Patients' results showing log10 transformed values for 24 hour urine (A) calcium excretion, (B) oxalate excretion, and (C) the calcium times oxalate product excretion. The five patients' results are represented by open circles, open squares, open diamonds, open triangles, and filled circles, respectively. In the interests of clarity, individual patients' results during each stage of the trial are simply stacked one above the other, rather than being depicted chronologically. Separate patients' results are depicted side by side to assist in interpreting the data. The solid lines represent the overall mean patients' results for periods 1, 2, 3, 4, and 5 of the trial. Error bars represent 1 SE about the mean. The broken lines represent overall mean values of patients from the reference population, whose urine samples were collected during the same weeks of the year as the trial patients. Significant differences between periods are only shown for the trial patients and were calculated by two way analysis of variance, followed by the Fisher LSD test.
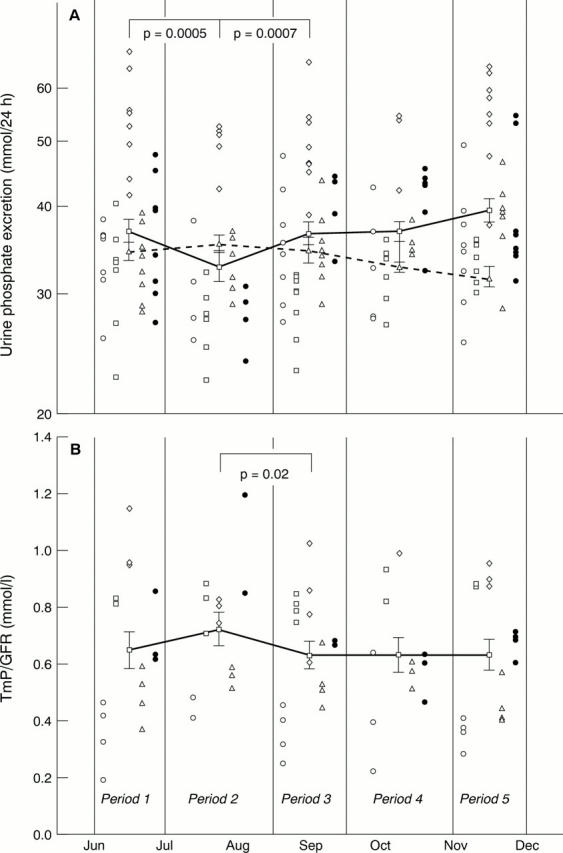
Figure 3 Pooled patients' results showing log10 transformed values for (A) 24 hour urine phosphate excretion and (B) renal tubular reabsorption of phosphate (TmP/GFR ratio). Individual patients' results are represented by open circles, open squares, open diamonds, open triangles, and filled circles, respectively. In the interests of clarity, individual patients' results during each stage of the trial are simply stacked one above the other, rather than being depicted chronologically. Separate patients' results are depicted side by side to assist in interpreting the data. The solid lines show the mean results for periods 1, 2, 3, 4, and 5 of the trial. The broken lines represent overall mean values of patients from the reference population, whose urine samples were collected during the same weeks of the year as the trial patients. Error bars represent 1 SE about the mean. Significant differences between periods are only shown for the trial patients and were calculated by two way analysis of variance, followed by the Fisher LSD test.
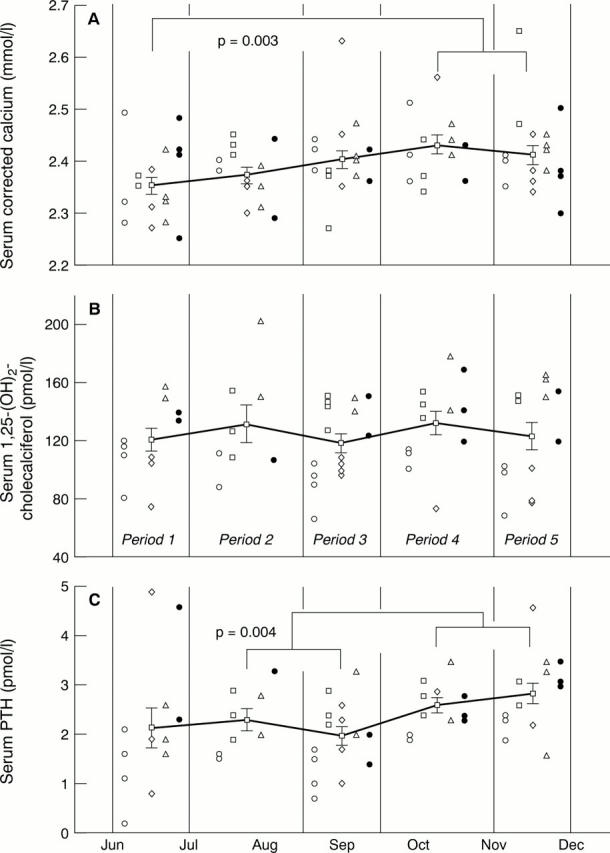
Figure 4 Pooled patients' results showing (A) serum calcium, (B) serum 1,25-(OH)2-cholecalciferol, and (C) serum parathyroid hormone (PTH). The five patients' results are represented by open circles, open squares, open diamonds, open triangles, and filled circles, respectively. In the interests of clarity, individual patients' results during each stage of the trial are simply stacked one above the other, rather than being depicted chronologically. Separate patients' results are depicted side by side to assist in interpreting the data. The solid lines show the mean results for periods 1, 2, 3, 4, and 5 of the trial. Error bars represent 1 SE about the mean. Significant differences between periods were calculated by two way analysis of variance, followed by the Fisher LSD test. For the purpose of statistical analysis, results for periods 2 and 3 (on calcium supplements) and periods 4 and 5 (off calcium supplements) were pooled.
Figure 5 Scatter diagram of log 24 hour urine oxalate excretion against log calcium excretion on data from a group of 1066 urine collections from patients on follow up for previous renal stone disease. The regression parameter associated with the regression line was 0.155 (95% confidence interval, 0.045 to 0.266; p = 0.006).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Arroyo M. V., Traba M. L., Rapado A., de la Piedra C., Torralbo M. Correlation between 1.25 dihydroxyvitamin D serum levels and fractional rate of intestinal calcium absorption in hypercalciuric nephrolithiasis. Role of phosphate. Urol Res. 1992;20(1):96–97. doi: 10.1007/BF00294348. [DOI] [PubMed] [Google Scholar]
- Berlin T., Björkhem I. Effect of calcium intake on serum levels of 25-hydroxyvitamin D3. Eur J Clin Invest. 1988 Feb;18(1):52–55. doi: 10.1111/j.1365-2362.1988.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Brändle E., Bernt U., Hautmann R. E. In situ characterization of oxalate transport across the basolateral membrane of the proximal tubule. Pflugers Arch. 1998 May;435(6):840–849. doi: 10.1007/s004240050592. [DOI] [PubMed] [Google Scholar]
- Cai Q., Hodgson S. F., Kao P. C., Lennon V. A., Klee G. G., Zinsmiester A. R., Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994 Jun 9;330(23):1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- Caspary W. F. Intestinal oxalate absorption. I. Absorption in vitro. Res Exp Med (Berl) 1977 Aug 16;171(1):13–24. doi: 10.1007/BF01851584. [DOI] [PubMed] [Google Scholar]
- Caudarella R., Rizzoli E., Buffa A., Bottura A., Stefoni S. Comparative study of the influence of 3 types of mineral water in patients with idiopathic calcium lithiasis. J Urol. 1998 Mar;159(3):658–663. [PubMed] [Google Scholar]
- Crook M., Swaminathan R. Disorders of plasma phosphate and indications for its measurement. Ann Clin Biochem. 1996 Sep;33(Pt 5):376–396. doi: 10.1177/000456329603300502. [DOI] [PubMed] [Google Scholar]
- Curhan G. C., Willett W. C., Rimm E. B., Stampfer M. J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993 Mar 25;328(12):833–838. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- Curhan G. C., Willett W. C., Speizer F. E., Spiegelman D., Stampfer M. J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997 Apr 1;126(7):497–504. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- Eaton S. B., Nelson D. A. Calcium in evolutionary perspective. Am J Clin Nutr. 1991 Jul;54(1 Suppl):281S–287S. doi: 10.1093/ajcn/54.1.281S. [DOI] [PubMed] [Google Scholar]
- Econs M. J., Drezner M. K. Tumor-induced osteomalacia--unveiling a new hormone. N Engl J Med. 1994 Jun 9;330(23):1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- Fraser D. R. Vitamin D. Lancet. 1995 Jan 14;345(8942):104–107. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Giannini S., Nobile M., Castrignano R., Pati T., Tasca A., Villi G., Pellegrini F., D'Angelo A. Possible link between vitamin D and hyperoxaluria in patients with renal stone disease. Clin Sci (Lond) 1993 Jan;84(1):51–54. doi: 10.1042/cs0840051. [DOI] [PubMed] [Google Scholar]
- Gross M., Kumar R. Physiology and biochemistry of vitamin D-dependent calcium binding proteins. Am J Physiol. 1990 Aug;259(2 Pt 2):F195–F209. doi: 10.1152/ajprenal.1990.259.2.F195. [DOI] [PubMed] [Google Scholar]
- Guillemant J., Guillemant S. Acute PTH response to oral calcium load and seasonal variation of vitamin D status in healthy young adult subjects. Eur J Clin Nutr. 1996 Jul;50(7):469–472. [PubMed] [Google Scholar]
- Hallson P. C., Kasidas G. P., Rose G. A. Urinary oxalate in summer and winter in normal subjects and in stone-forming patients with idiopathic hypercalciuria, both untreated and treated with thiazide and/or cellulose phosphate. Urol Res. 1976;4(4):169–173. doi: 10.1007/BF00262350. [DOI] [PubMed] [Google Scholar]
- Hallson P. C., Rose G. A. Seasonal variations in urinary crystals. Br J Urol. 1977 Aug;49(4):277–284. doi: 10.1111/j.1464-410x.1977.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Ho S. C., Mac Donald D., Chan C., Fan Y. K., Chan S. S., Swaminathan R. Determinants of serum 1,25-dihydroxyvitamin D concentration in healthy premenopausal subjects. Clin Chim Acta. 1994 Oct 14;230(1):21–33. doi: 10.1016/0009-8981(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Goodman H. O., Assimos D. G. Dietary oxalate and its intestinal absorption. Scanning Microsc. 1995;9(4):1109–1120. [PubMed] [Google Scholar]
- Johnson C. M., Wilson D. M., O'Fallon W. M., Malek R. S., Kurland L. T. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int. 1979 Nov;16(5):624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- Juuti M., Heinonen O. P., Alhava E. M. Seasonal variation in urinary excretion of calcium, oxalate, magnesium and phosphate on free and standard mineral diet in men with urolithiasis. Scand J Urol Nephrol. 1981;15(2):137–141. doi: 10.3109/00365598109179590. [DOI] [PubMed] [Google Scholar]
- Kato T., Yanagawa M., Hioki T., Sakurai M., Yamakawa K., Araki T., Yamamoto I., Arima K., Tochigi H., Kawamura J. [Dietary control for out-patients in urinary stone clinic]. Hinyokika Kiyo. 1993 Jul;39(7):593–598. [PubMed] [Google Scholar]
- Kemp G. J., Blumsohn A., Morris B. W. Circadian changes in plasma phosphate concentration, urinary phosphate excretion, and cellular phosphate shifts. Clin Chem. 1992 Mar;38(3):400–402. [PubMed] [Google Scholar]
- Lemann J., Jr, Pleuss J. A., Worcester E. M., Hornick L., Schrab D., Hoffmann R. G. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996 Jan;49(1):200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- Leonetti F., Dussol B., Berthezene P., Thirion X., Berland Y. Dietary and urinary risk factors for stones in idiopathic calcium stone formers compared with healthy subjects. Nephrol Dial Transplant. 1998 Mar;13(3):617–622. doi: 10.1093/ndt/13.3.617. [DOI] [PubMed] [Google Scholar]
- Liebman M., Chai W. Effect of dietary calcium on urinary oxalate excretion after oxalate loads. Am J Clin Nutr. 1997 May;65(5):1453–1459. doi: 10.1093/ajcn/65.5.1453. [DOI] [PubMed] [Google Scholar]
- Lloyd S. E., Pearce S. H., Fisher S. E., Steinmeyer K., Schwappach B., Scheinman S. J., Harding B., Bolino A., Devoto M., Goodyer P. A common molecular basis for three inherited kidney stone diseases. Nature. 1996 Feb 1;379(6564):445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- Madsen K. L., Tavernini M. M., Yachimec C., Mendrick D. L., Alfonso P. J., Buergin M., Olsen H. S., Antonaccio M. J., Thomson A. B., Fedorak R. N. Stanniocalcin: a novel protein regulating calcium and phosphate transport across mammalian intestine. Am J Physiol. 1998 Jan;274(1 Pt 1):G96–102. doi: 10.1152/ajpgi.1998.274.1.G96. [DOI] [PubMed] [Google Scholar]
- Marshall R. W., Cochran M., Robertson W. G., Hodgkinson A., Nordin B. E. The relation between the concentration of calcium salts in the urine and renal stone composition in patients with calcium-containing renal stones. Clin Sci. 1972 Sep;43(3):433–441. doi: 10.1042/cs0430433. [DOI] [PubMed] [Google Scholar]
- Masai M., Ito H., Kotake T. Effect of dietary intake on urinary oxalate excretion in calcium renal stone formers. Br J Urol. 1995 Dec;76(6):692–696. doi: 10.1111/j.1464-410x.1995.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Messa P., Marangella M., Paganin L., Codardini M., Cruciatti A., Turrin D., Filiberto Z., Mioni G. Different dietary calcium intake and relative supersaturation of calcium oxalate in the urine of patients forming renal stones. Clin Sci (Lond) 1997 Sep;93(3):257–263. doi: 10.1042/cs0930257. [DOI] [PubMed] [Google Scholar]
- Norlin A., Lindell B., Granberg P. O., Lindvall N. Urolithiasis. A study of its frequency. Scand J Urol Nephrol. 1976;10(2):150–153. doi: 10.3109/00365597609179677. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Friedlander E. J., Henry H. L. Determination of the rates of synthesis and degradation of vitamin D-dependent chick intestinal and renal calcium-binding proteins. Arch Biochem Biophys. 1981 Feb;206(2):305–317. doi: 10.1016/0003-9861(81)90096-5. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Circadian rhythms of urinary saturation levels of calcium oxalate and calcium phosphate in normal male individuals. Hinyokika Kiyo. 1993 Sep;39(9):785–789. [PubMed] [Google Scholar]
- Payne R. B. Renal tubular reabsorption of phosphate (TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998 Mar;35(Pt 2):201–206. doi: 10.1177/000456329803500203. [DOI] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Murphy M. M., Morris R. C., Jr Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986 Jan;77(1):7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M., Roux J. O., Bourelly F., Boularan A. M., Guiter J., Monnier L. Circadian variations in the risk of urinary calcium oxalate stone formation. Br J Urol. 1994 Sep;74(3):294–297. doi: 10.1111/j.1464-410x.1994.tb16613.x. [DOI] [PubMed] [Google Scholar]
- Robert M., Roux J. O., Bourelly F., Boularan A. M., Guiter J., Monnier L. Circadian variations in the risk of urinary calcium oxalate stone formation. Br J Urol. 1994 Sep;74(3):294–297. doi: 10.1111/j.1464-410x.1994.tb16613.x. [DOI] [PubMed] [Google Scholar]
- Robertson W. G., Gallagher J. C., Marshall D. H., Peacock M., Nordin B. E. Seasonal variations in urinary excretion of calcium. Br Med J. 1974 Nov 23;4(5942):436–437. doi: 10.1136/bmj.4.5942.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson W. G., Peacock M., Marshall R. W., Speed R., Nordin B. E. Seasonal variations in the composition of urine in relation to calcium stone-formation. Clin Sci Mol Med. 1975 Dec;49(6):597–602. doi: 10.1042/cs0490597. [DOI] [PubMed] [Google Scholar]
- Robertson W. G., Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron. 1980;26(3):105–110. doi: 10.1159/000181963. [DOI] [PubMed] [Google Scholar]
- Rose G. A., Westbury E. J. Seasonal and geographic variations in urinary composition in England, Scotland and Wales. Urol Res. 1979 Sep;7(3):235–240. doi: 10.1007/BF00257211. [DOI] [PubMed] [Google Scholar]
- Sierakowski R., Finlayson B., Landes R. R., Finlayson C. D., Sierakowski N. The frequency of urolithiasis in hospital discharge diagnoses in the United States. Invest Urol. 1978 May;15(6):438–441. [PubMed] [Google Scholar]
- Taylor W. H. Renal calculi and self-medication with multivitamin preparations containing vitamin D. Clin Sci. 1972 Apr;42(4):515–522. doi: 10.1042/cs0420515. [DOI] [PubMed] [Google Scholar]
- Vahlensieck E. W., Bach D., Hesse A. Circadian rhythm of lithogenic substances in the urine. Urol Res. 1982;10(4):195–203. doi: 10.1007/BF00255944. [DOI] [PubMed] [Google Scholar]
- Veenhuizen L., Donckerwolcke R. A. Role of 1,25-dihydroxyvitamin D production in idiopathic hypercalciuria. Child Nephrol Urol. 1991;11(2):69–73. [PubMed] [Google Scholar]
- Verkoelen C. F., Romijn J. C., de Bruijn W. C., Boevé E. R., Cao L. C., Schröder F. H. Absence of a transcellular oxalate transport mechanism in LLC-PK1 and MDCK cells cultured on porous supports. Scanning Microsc. 1993 Sep;7(3):1031–1040. [PubMed] [Google Scholar]
- Walton R. J., Bijvoet O. L. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975 Aug 16;2(7929):309–310. doi: 10.1016/s0140-6736(75)92736-1. [DOI] [PubMed] [Google Scholar]
- Williams C. P., Child D. F., Hudson P. R., Soysa L. D., Davies G. K., Davies M. G., De Bolla A. R. Inappropriate phosphate excretion in idiopathic hypercalciuria: the key to a common cause and future treatment? J Clin Pathol. 1996 Nov;49(11):881–888. doi: 10.1136/jcp.49.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida O., Okada Y. Epidemiology of urolithiasis in Japan: a chronological and geographical study. Urol Int. 1990;45(2):104–111. doi: 10.1159/000281680. [DOI] [PubMed] [Google Scholar]