Abstract
Background/Aims—Many regimens used in the treatment of childhood acute lymphoblastic leukaemia (ALL) include Daunorubicin or Etoposide, which act as topoisomerase poisons. It has been suggested that there may be a relation between topoisomerase expression and response to topoisomerase poisons, based mainly on results from in vitro studies. Therefore, the aim of this study was to investigate this relation in a clinical setting and determine whether topoisomerase IIα and IIß might be of predictive value in ALL.
Methods—Cellular expression of topoisomerases IIα and IIß was assessed in 177 cases of ALL by immunohistochemistry using monoclonal antibodies to the two enzymes. The percentages of cell nuclei showing positive staining for topoisomerase IIα and IIß expression were assessed.
Results—Taking the series as a whole, a clear separation of survival curves was seen with the established prognostic markers white blood cell (WBC) count, CD10 status, and sex. However, topoisomerase IIα and IIß expression showed no relation to survival. No association was found between the topoisomerases and the prognostic markers CD10 and WBC count; however, topoisomerase IIα expression was found to be related to sex, with expression being lower in girls (p = 0.002).
Conclusions—These results suggest that the response to topoisomerase poisons cannot be predicted by the assessment of topoisomerase IIα and IIß expression as defined by immunohistochemistry.
Key Words: topoisomerase • leukaemia • prognosis • immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (206.1 KB).
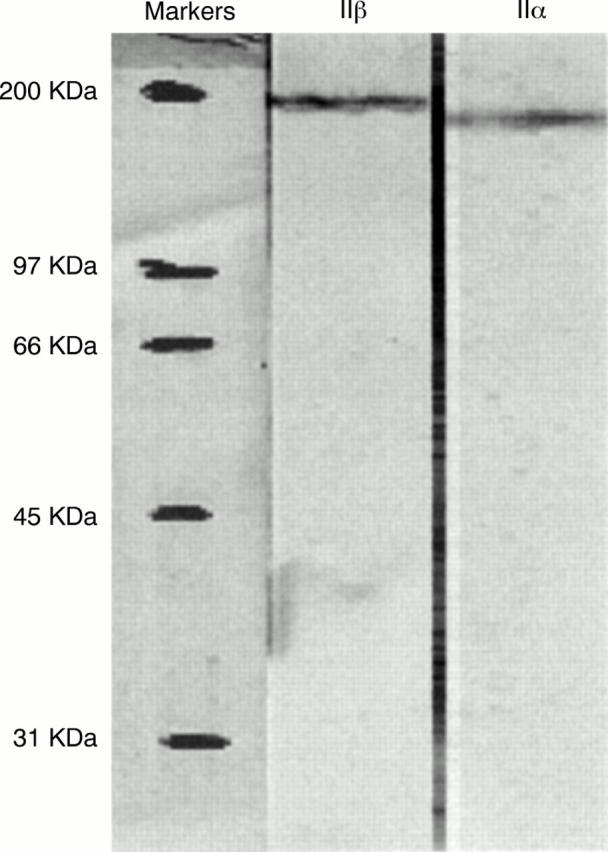
Figure 1 Western blot showing immunoreactivity of TOPO2ß-8.71 with a 180 kDa protein (left) and NCL-TOPOIIA with a 170 kDa protein (right) using a lysate of a RAJI cell line.
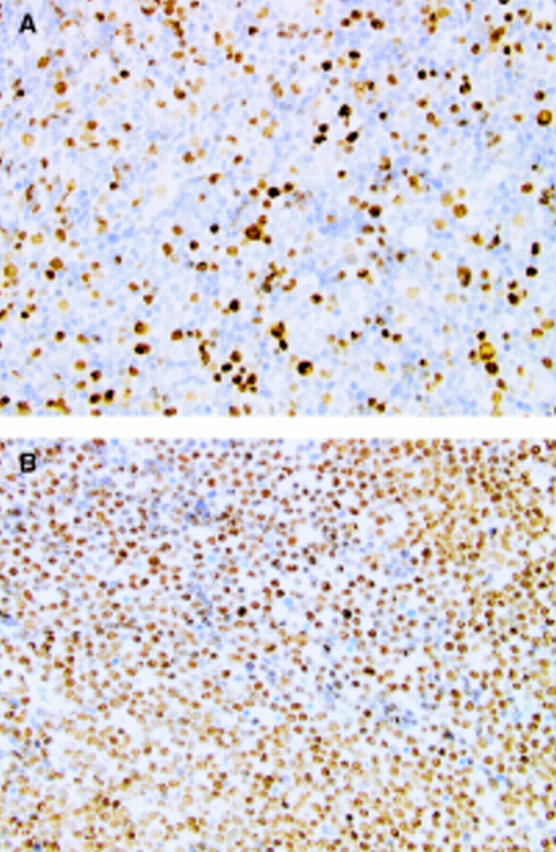
Figure 2 Immunohistochemical staining of (A) topoisomerase IIα and (B) topoisomerase IIß in an acute lymphoblastic leukaemia bone marrow trephine showing extensive infiltration. Note intensive staining of tumour cell nuclei in both instances.
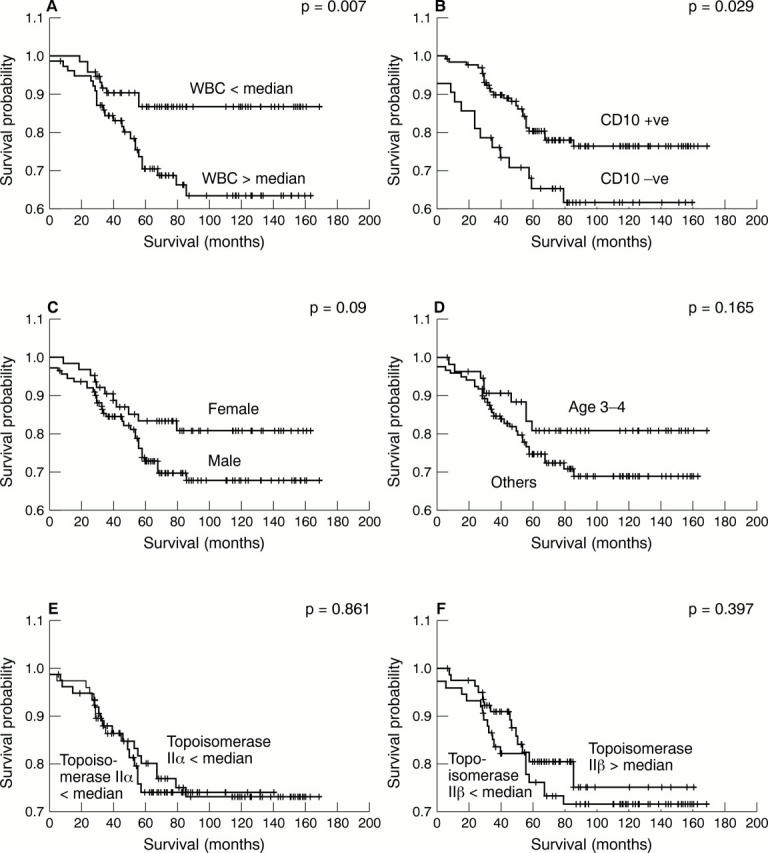
Figure 3 Kaplain Meier survival analysis for all patients (n = 160) according to (A) presenting white blood cell (WBC) count, (B) CD10, (C) sex, (D) age, (E) topoisomerase IIα, and (F) topoisomerase IIß.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J., Handgretinger R., Dopfer R., Klingebiel T., Niethammer D., Gekeler V. Expression of mdr1, mrp, topoisomerase II alpha/beta, and cyclin A in primary or relapsed states of acute lymphoblastic leukaemias. Br J Haematol. 1995 Feb;89(2):356–363. doi: 10.1111/j.1365-2141.1995.tb03312.x. [DOI] [PubMed] [Google Scholar]
- Brown G. A., McPherson J. P., Gu L., Hedley D. W., Toso R., Deuchars K. L., Freedman M. H., Goldenberg G. J. Relationship of DNA topoisomerase II alpha and beta expression to cytotoxicity of antineoplastic agents in human acute lymphoblastic leukemia cell lines. Cancer Res. 1995 Jan 1;55(1):78–82. [PubMed] [Google Scholar]
- Buchanan CD, Chun SB. Simple predictive model for flavor production in hadronization. Phys Rev Lett. 1987 Nov 2;59(18):1997–2000. doi: 10.1103/PhysRevLett.59.1997. [DOI] [PubMed] [Google Scholar]
- Béné M. C., Faure G. C. CD10 in acute leukemias. GEIL (Groupe d'Etude Immunologique des Leucémies). Haematologica. 1997 Mar-Apr;82(2):205–210. [PubMed] [Google Scholar]
- Cassano W. F., Eskenazi A. E., Frantz C. N. Therapy for childhood acute lymphoblastic leukemia. Curr Opin Oncol. 1993 Jan;5(1):42–52. [PubMed] [Google Scholar]
- Cattan A. R., Levett D., Douglas E. A., Middleton P. G., Taylor P. R. Method for quantifying expression of functionally active topoisomerase II in patients with leukaemia. J Clin Pathol. 1996 Oct;49(10):848–852. doi: 10.1136/jcp.49.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessells J. M., Bailey C., Richards S. M. Intensification of treatment and survival in all children with lymphoblastic leukaemia: results of UK Medical Research Council trial UKALL X. Medical Research Council Working Party on Childhood Leukaemia. Lancet. 1995 Jan 21;345(8943):143–148. doi: 10.1016/s0140-6736(95)90164-7. [DOI] [PubMed] [Google Scholar]
- Chessells J. M., Richards S. M., Bailey C. C., Lilleyman J. S., Eden O. B. Gender and treatment outcome in childhood lymphoblastic leukaemia: report from the MRC UKALL trials. Br J Haematol. 1995 Feb;89(2):364–372. doi: 10.1111/j.1365-2141.1995.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Davies S. M., Robson C. N., Davies S. L., Hickson I. D. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988 Nov 25;263(33):17724–17729. [PubMed] [Google Scholar]
- Fernandes D. J., Danks M. K., Beck W. T. Decreased nuclear matrix DNA topoisomerase II in human leukemia cells resistant to VM-26 and m-AMSA. Biochemistry. 1990 May 1;29(17):4235–4241. doi: 10.1021/bi00469a028. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Chresta C. M., Davies S. M., Walker M. C., Harris A. L., Hartley J. A., Masters J. R., Hickson I. D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991 Dec 15;51(24):6592–6595. [PubMed] [Google Scholar]
- Greaves M. F., Brown G., Rapson N. T., Lister T. A. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975 May;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Holden J. A., Rolfson D. H., Wittwer C. T. Human DNA topoisomerase II: evaluation of enzyme activity in normal and neoplastic tissues. Biochemistry. 1990 Feb 27;29(8):2127–2134. doi: 10.1021/bi00460a024. [DOI] [PubMed] [Google Scholar]
- Järvinen T. A., Kononen J., Pelto-Huikko M., Isola J. Expression of topoisomerase IIalpha is associated with rapid cell proliferation, aneuploidy, and c-erbB2 overexpression in breast cancer. Am J Pathol. 1996 Jun;148(6):2073–2082. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Karp J. E., Jones R. J., Miller C. B., Schneider E., Zwelling L. A., Cowan K., Wendel K., Burke P. J. Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood. 1994 Jan 15;83(2):517–530. [PubMed] [Google Scholar]
- Klumper E., Giaccone G., Pieters R., Broekema G., van Ark-Otte J., van Wering E. R., Kaspers G. J., Veerman A. J. Topoisomerase II alpha gene expression in childhood acute lymphoblastic leukemia. Leukemia. 1995 Oct;9(10):1653–1660. [PubMed] [Google Scholar]
- Lodge A. J., Anderson J. J., Ng S. W., Fenwick F., Steward M., Haugk B., Horne C. H., Angus B. Expression of topoisomerase IIIalpha in normal and neoplastic tissues determined by immunohistochemistry using a novel monoclonal antibody. Br J Cancer. 2000 Aug;83(4):498–505. doi: 10.1054/bjoc.2000.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohri A., Reuter J., Gudat F., Herrmann R. Topoisomerase II alpha mRNA and tumour cell proliferation in non-Hodgkin's lymphoma. J Clin Pathol. 1997 Jan;50(1):22–26. doi: 10.1136/jcp.50.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna S. L., West R. R., Whittaker J. A., Padua R. A., Holmes J. A. Topoisomerase II alpha expression in acute myeloid leukaemia and its relationship to clinical outcome. Leukemia. 1994 Sep;8(9):1498–1502. [PubMed] [Google Scholar]
- Richards S., Burrett J., Hann I., Chessells J., Hill F., Bailey C. Improved survival with early intensification: combined results from the Medical Research Council childhood ALL randomised trials, UKALL X and UKALL XI. Medical Research Council Working Party on Childhood Leukaemia. Leukemia. 1998 Jul;12(7):1031–1036. doi: 10.1038/sj.leu.2401065. [DOI] [PubMed] [Google Scholar]
- Sullivan D. M., Latham M. D., Ross W. E. Proliferation-dependent topoisomerase II content as a determinant of antineoplastic drug action in human, mouse, and Chinese hamster ovary cells. Cancer Res. 1987 Aug 1;47(15):3973–3979. [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Towatari M., Adachi K., Marunouchi T., Saito H. Evidence for a critical role of DNA topoisomerase IIalpha in drug sensitivity revealed by inducible antisense RNA in a human leukaemia cell line. Br J Haematol. 1998 Jun;101(3):548–551. doi: 10.1046/j.1365-2141.1998.00713.x. [DOI] [PubMed] [Google Scholar]
- Woessner R. D., Mattern M. R., Mirabelli C. K., Johnson R. K., Drake F. H. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991 Apr;2(4):209–214. [PubMed] [Google Scholar]
- Yabuki N., Sasano H., Kato K., Ohara S., Toyota T., Nagura H., Miyaike M., Nozaki N., Kikuchi A. Immunohistochemical study of DNA topoisomerase II in human gastric disorders. Am J Pathol. 1996 Sep;149(3):997–1007. [PMC free article] [PubMed] [Google Scholar]


