Abstract
Aims—To clarify p21waf1/cip1 expression in sinonasal lesions.
Methods—Archived surgical specimens from 38 patients were investigated by means of immunohistochemistry. p21waf1/cip1 staining was evaluated in the different layers of the epithelium. In addition, human papillomavirus (HPV) infection and p53 protein overexpression were assessed and correlated with p21waf1/cip1 expression.
Results—p21waf1/cip1 staining was negative in non-papillomatous nasal mucosa. HPV infection and p53 protein overexpression were not seen. Sixteen of 20 inverted papillomas showed p21waf1/cip1 expression. HPV infection was found in 16 cases and p53 protein overexpression was present in 13 specimens. Expression of p21waf1/cip1 was restricted to surface cells in five cases, but involved basal/parabasal cells in 11 specimens. Immunoreactivity for p21waf1/cip1 in basal/parabasal cells colocalised with p53 protein overexpression. Enhanced expression rates for p21waf1/cip1 were seen in transitional and squamous epithelium compared with columnar epithelium. p21waf1/cip1 expression involved only surface cells in cylindrical cell papillomas. HPV infection and p53 protein overexpression were detected in all specimens. One of five squamous cell carcinomas showed p21waf1/cip1 expression. HPV infection was seen in two cases, and all carcinomas showed p53 protein overexpression.
Conclusions—Expression of p21waf1/cip1 is associated with terminal differentiation in surface cells in inverted papillomas and cylindrical cell papillomas, but not in non-papillomatous nasal mucosa. Overexpression of p53 protein colocalises with p21waf1/cip1 expression in basal/parabasal cells in inverted papillomas but not in cylindrical cell papillomas. Expression of p21waf1/cip1 in squamous cell carcinomas involves a subset of tumours with p53 protein overexpression.
Key Words: p21waf1/cip1 • nasal mucosa • sinonasal papillomas • squamous cell carcinomas
Full Text
The Full Text of this article is available as a PDF (241.3 KB).
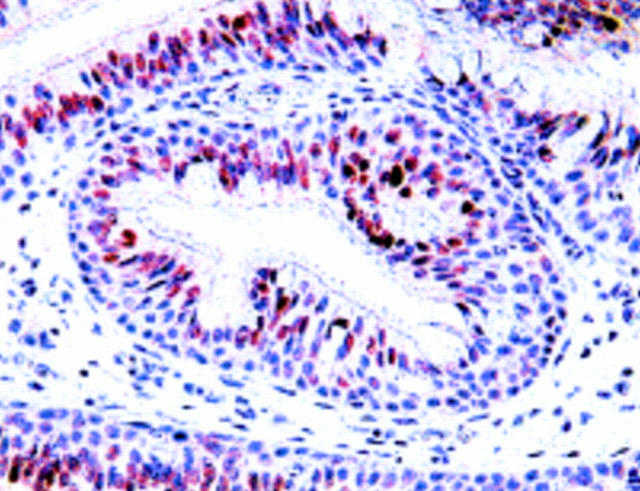
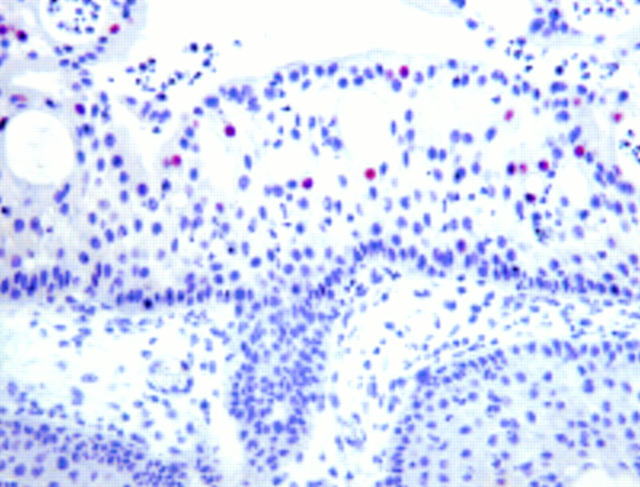
Figure 1 Columnar epithelium in the invaginating portion of an inverted papilloma from a 45 year old man. Positive staining for p21waf1/cip1 is restricted to surface cells. Negative staining for p21waf1/cip1 in basal/parabasal cells, although human papillomavirus infection and p53 protein overexpression were present in this specimen.
Figure 2 Squamous epithelium in an inverted papilloma (same specimen as shown in fig 1). Note abundant immunoreactivity for p21waf1/cip1 in basal/parabasal cells. In this case, positive staining in surface cells only (fig 1) together with positive staining in basal/parabasal cells only (fig 4) were found in different regions within the same specimen.
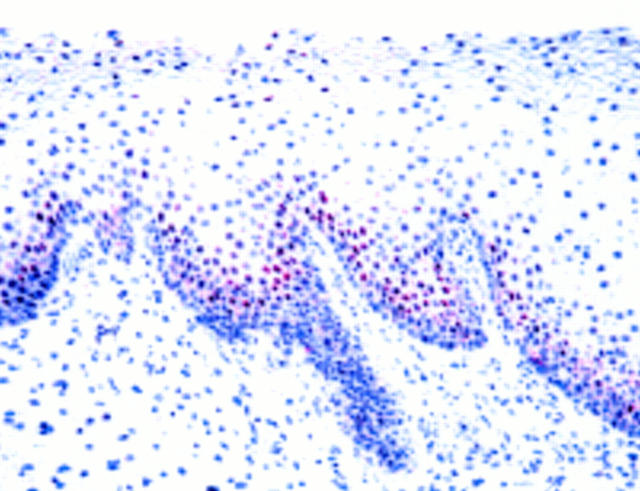
Figure 3 Transitional epithelium in an inverted papilloma from a 25 year old man. Abundant immunoreactivity for p53 protein is present in the lower half of the epithelium. Human papillomavirus infection was demonstrated in this specimen.
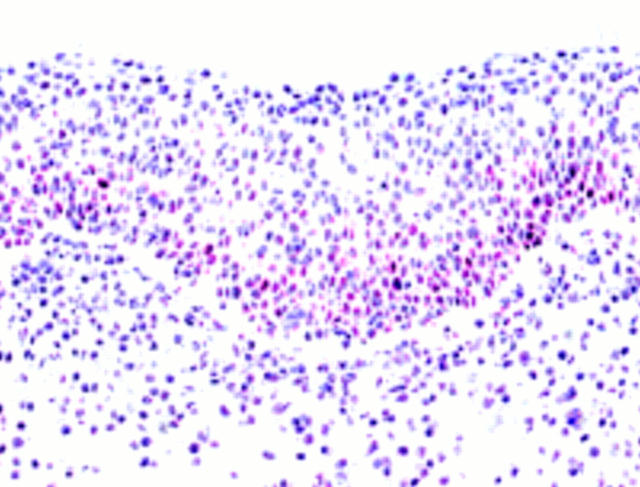
Figure 4 Immunoreactivity for p21waf1/cip1 in a serial slide from the same lesion shown in fig 3. Note colocalisation of positive staining results for p53 protein and p21waf1/cip1 between figs 2 and 3.
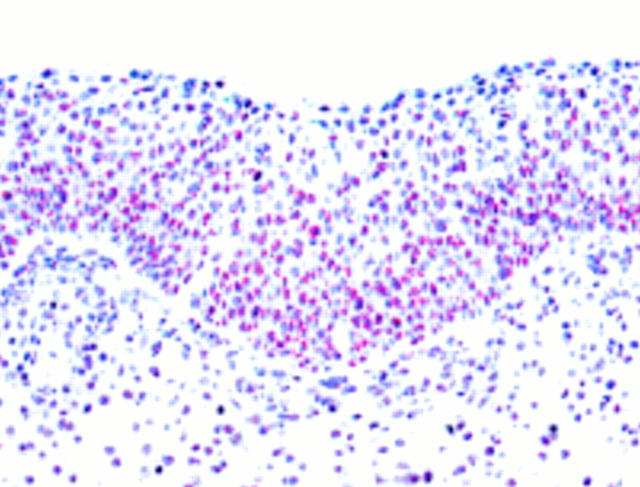
Figure 5 Cylindrical cell papilloma from a 54 year old man. Positive staining for p21waf1/cip1 is seen only in suprabasal and surface cells of the epithelium.
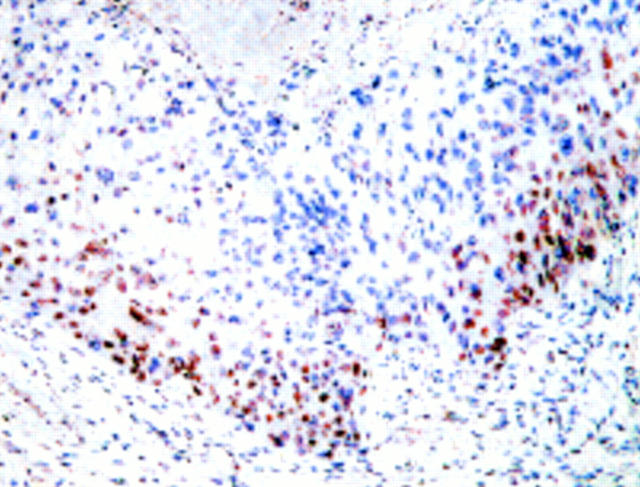
Figure 6 Squamous cell carcinoma associated with an inverted papilloma from a 61 year old man. Note immunoreactivity for p21waf1/cip1 on the invasion front of the tumour. Necrotic areas of the tumour were negative for p21waf1/cip1 expression (upper part of the picture). Human papillomavirus infection was not detected in this specimen. Abundant overexpression of the p53 protein was seen in this tumour.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Mathur M., Shukla N. K., Ralhan R. Expression of cyclin dependent kinase inhibitor p21waf1/cip1 in premalignant and malignant oral lesions: relationship with p53 status. Oral Oncol. 1998 Sep;34(5):353–360. doi: 10.1016/s1368-8375(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Barbareschi M., Caffo O., Doglioni C., Fina P., Marchetti A., Buttitta F., Leek R., Morelli L., Leonardi E., Bevilacqua G. p21WAF1 immunohistochemical expression in breast carcinoma: correlations with clinicopathological data, oestrogen receptor status, MIB1 expression, p53 gene and protein alterations and relapse-free survival. Br J Cancer. 1996 Jul;74(2):208–215. doi: 10.1038/bjc.1996.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia K., Fan S., Spangler G., Weintraub M., O'Connor P. M., Judde J. G., Magrath I. A mutant p21 cyclin-dependent kinase inhibitor isolated from a Burkitt's lymphoma. Cancer Res. 1995 Apr 1;55(7):1431–1435. [PubMed] [Google Scholar]
- Bielamowicz S., Calcaterra T. C., Watson D. Inverting papilloma of the head and neck: the UCLA update. Otolaryngol Head Neck Surg. 1993 Jul;109(1):71–76. doi: 10.1177/019459989310900113. [DOI] [PubMed] [Google Scholar]
- Caffo O., Doglioni C., Veronese S., Bonzanini M., Marchetti A., Buttitta F., Fina P., Leek R., Morelli L., Palma P. D. Prognostic value of p21(WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res. 1996 Sep;2(9):1591–1599. [PubMed] [Google Scholar]
- Cagle P. T., Brown R. W., Lebovitz R. M. p53 immunostaining in the differentiation of reactive processes from malignancy in pleural biopsy specimens. Hum Pathol. 1994 May;25(5):443–448. doi: 10.1016/0046-8177(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Califano J., Koch W., Sidransky D., Westra W. H. Inverted sinonasal papilloma : a molecular genetic appraisal of its putative status as a Precursor to squamous cell carcinoma. Am J Pathol. 2000 Jan;156(1):333–337. doi: 10.1016/S0002-9440(10)64734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana S. M., Zwiebel N., Cocker R., McCormick S. A., Eberle R. C., Lazarus P. p53 alteration and human papilloma virus infection in paranasal sinus cancer. Cancer. 1997 Apr 1;79(7):1320–1328. [PubMed] [Google Scholar]
- Datto M. B., Li Y., Panus J. F., Howe D. J., Xiong Y., Wang X. F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto F., Topley G., Calautti E., Hsiao J., Ong L., Seth P. K., Dotto G. P. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998 May 15;280(5366):1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Redston M. S., Yeo C. J., Kern S. E., Hruban R. H. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol. 1995 Oct;147(4):884–888. [PMC free article] [PubMed] [Google Scholar]
- Doglioni C., Pelosio P., Laurino L., Macri E., Meggiolaro E., Favretti F., Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996 Jul;179(3):248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Fang S. Y., Yan J. J., Ohyama M. Assessment of p53 protein expression in normal mucosa and benign and malignant lesions of the nasal cavity. Oncology. 1998 Mar-Apr;55(2):168–173. doi: 10.1159/000011852. [DOI] [PubMed] [Google Scholar]
- Fouret P., Dabit D., Sibony M., Alili D., Commo F., Saint-Guily J. L., Callard P. Expression of p53 protein related to the presence of human papillomavirus infection in precancer lesions of the larynx. Am J Pathol. 1995 Mar;146(3):599–604. [PMC free article] [PubMed] [Google Scholar]
- Franzmann M. B., Buchwald C., Jacobsen G. K., Lindeberg H. Expression of p53 in normal nasal mucosa and in sinonasal papillomas with and without associated carcinoma and the relation to human papillomavirus (HPV). Cancer Lett. 1998 Jun 19;128(2):161–164. doi: 10.1016/s0304-3835(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Gervais J. L., Seth P., Zhang H. Cleavage of CDK inhibitor p21(Cip1/Waf1) by caspases is an early event during DNA damage-induced apoptosis. J Biol Chem. 1998 Jul 24;273(30):19207–19212. doi: 10.1074/jbc.273.30.19207. [DOI] [PubMed] [Google Scholar]
- Hwang C. S., Yang H. S., Hong M. K. Detection of human papillomavirus (HPV) in sinonasal inverted papillomas using polymerase chain reaction (PCR). Am J Rhinol. 1998 Sep-Oct;12(5):363–366. doi: 10.2500/105065898780182499. [DOI] [PubMed] [Google Scholar]
- Ingle R. R., Setzen G., Koltai P. J., Monte D., Pastore J., Jennings T. A. p53 protein expression in benign lesions of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 1997 Mar;123(3):297–300. doi: 10.1001/archotol.1997.01900030071009. [DOI] [PubMed] [Google Scholar]
- Kamei I., Obayashi S., Nakagawa M., Nishibayashi H., Kuwata T., Hyotani G., Yabumoto M., Kuriyama T., Itakura T., Komai N. [When do strokes occur?--analysis of diurnal variation and activity during the onset]. No Shinkei Geka. 1998 Nov;26(11):991–998. [PubMed] [Google Scholar]
- Lawson W., Ho B. T., Shaari C. M., Biller H. F. Inverted papilloma: a report of 112 cases. Laryngoscope. 1995 Mar;105(3 Pt 1):282–288. doi: 10.1288/00005537-199503000-00011. [DOI] [PubMed] [Google Scholar]
- Li Y., Jenkins C. W., Nichols M. A., Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994 Aug;9(8):2261–2268. [PubMed] [Google Scholar]
- Michaels L., Young M. Histogenesis of papillomas of the nose and paranasal sinuses. Arch Pathol Lab Med. 1995 Sep;119(9):821–826. [PubMed] [Google Scholar]
- Mirza N., Montone K., Sato Y., Kroger H., Kennedy D. W. Identification of p53 and human papilloma virus in Schneiderian papillomas. Laryngoscope. 1998 Apr;108(4 Pt 1):497–501. doi: 10.1097/00005537-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Missero C., Calautti E., Eckner R., Chin J., Tsai L. H., Livingston D. M., Dotto G. P. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng I. O., Lam K. Y., Ng M., Regezi J. A. Expression of p21/waf1 in oral squamous cell carcinomas--correlation with p53 and mdm2 and cellular proliferation index. Oral Oncol. 1999 Jan;35(1):63–69. doi: 10.1016/s1368-8375(98)00083-9. [DOI] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Raschka L. B. The incubus syndrome. A variant of erotomania. Can J Psychiatry. 1979 Oct;24(6):549–553. doi: 10.1177/070674377902400612. [DOI] [PubMed] [Google Scholar]
- Schmidt-Grimminger D. C., Wu X., Jian Y., Broker T. R., Chow L. T. Post-transcriptional induction of p21cip1 protein in condylomata and dysplasias is inversely related to human papillomavirus activities. Am J Pathol. 1998 Apr;152(4):1015–1024. [PMC free article] [PubMed] [Google Scholar]
- Schwerer M. J., Kraft K., Baczako K. Structural changes in the gastric foveolar epithelium in Helicobacter pylori-positive gastritis revealed by keratin immunohistochemistry. Hum Pathol. 1997 Nov;28(11):1260–1267. doi: 10.1016/s0046-8177(97)90199-4. [DOI] [PubMed] [Google Scholar]
- Shepherd P., Lunny D., Brookes R., Palmer T., McCance D. The detection of human papillomaviruses in cervical biopsies by immunohistochemistry and in situ hybridization. Scand J Immunol Suppl. 1992;11:69–74. doi: 10.1111/j.1365-3083.1992.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Steinman R. A., Hoffman B., Iro A., Guillouf C., Liebermann D. A., el-Houseini M. E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994 Nov;9(11):3389–3396. [PubMed] [Google Scholar]
- Upadhyay S., Li G., Liu H., Chen Y. Q., Sarkar F. H., Kim H. R. bcl-2 suppresses expression of p21WAF1/CIP1 in breast epithelial cells. Cancer Res. 1995 Oct 15;55(20):4520–4524. [PubMed] [Google Scholar]
- Vidal M. J., Loganzo F., Jr, de Oliveira A. R., Hayward N. K., Albino A. P. Mutations and defective expression of the WAF1 p21 tumour-suppressor gene in malignant melanomas. Melanoma Res. 1995 Aug;5(4):243–250. doi: 10.1097/00008390-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Weinberg W. C., Fernandez-Salas E., Morgan D. L., Shalizi A., Mirosh E., Stanulis E., Deng C., Hennings H., Yuspa S. H. Genetic deletion of p21WAF1 enhances papilloma formation but not malignant conversion in experimental mouse skin carcinogenesis. Cancer Res. 1999 May 1;59(9):2050–2054. [PubMed] [Google Scholar]
- Wu Y., Huang H., Miner Z., Kulesz-Martin M. Activities and response to DNA damage of latent and active sequence-specific DNA binding forms of mouse p53. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):8982–8987. doi: 10.1073/pnas.94.17.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yook J. I., Kim J. Expression of p21WAF1/CIP1 is unrelated to p53 tumour suppressor gene status in oral squamous cell carcinomas. Oral Oncol. 1998 May;34(3):198–203. doi: 10.1016/s1368-8375(97)00091-2. [DOI] [PubMed] [Google Scholar]
- Zumbach K., Hoffmann M., Kahn T., Bosch F., Gottschlich S., Görögh T., Rudert H., Pawlita M. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int J Cancer. 2000 Mar 15;85(6):815–818. doi: 10.1002/(sici)1097-0215(20000315)85:6<815::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- de Jong J. S., van Diest P. J., Michalides R. J., Baak J. P. Concerted overexpression of the genes encoding p21 and cyclin D1 is associated with growth inhibition and differentiation in various carcinomas. Mol Pathol. 1999 Apr;52(2):78–83. doi: 10.1136/mp.52.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]
- el-Deiry W. S. p21/p53, cellular growth control and genomic integrity. Curr Top Microbiol Immunol. 1998;227:121–137. doi: 10.1007/978-3-642-71941-7_6. [DOI] [PubMed] [Google Scholar]