Abstract
Aims—The use of the H score (involving the assessment of intensity and distribution of positivity) on sections stained for the oestrogen receptor (ER) by immunocytochemistry (ICC) allows different samples to be compared and detailed correlations to be made between hormone receptor expression and morphology. This study assessed the reliability of core biopsy in predicting ER expression in the same tumour excised later at treatment. The distribution of ER within excised tumours was investigated.
Methods—The distribution of ER positivity was investigated in 51 diagnostic core biopsies and across the diameter of 51 subsequently excised tumours in a field by field (magnification, x40; field diameter, 0.4 mm) assessment using the semiquantitive H scoring system.
Results—The ER H score in diagnostic core biopsy was significantly higher (p = 0.05, paired rank test; overall mean, 130; n = 51) than the mean in the corresponding excised tumour (mean, 110; n = 51). There was a significant downward trend in ER positivity from the periphery of tumours towards the centre (p = 0.001). The reduction of ER positivity was 6 H score units (2%)/mm. If core biopsies were orientated with the tumour edge at one end no change in ER positivity with field number along the length of the core could be demonstrated.
Conclusions—ER estimation in core biopsies correlated well with expression in tumours but ER expression was higher in the core biopsies than in the excised tumours. ER expression was higher at the periphery of tumours than at the centre. The higher ER expression in cores may reflect the higher chance of sampling the peripheral part of a tumour using a needle core.
Key Words: breast carcinoma • oestrogen receptor • core biopsy
Full Text
The Full Text of this article is available as a PDF (125.8 KB).
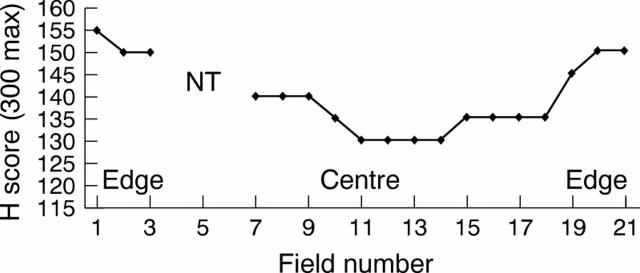
Figure 1 Plot of field by field H scores across the diameter of a single whole excised tumour showing reduced immunopositivity for oestrogen receptors (ER) in the centre. No tumour was present for assessment in fields 4, 5, and 6 (NT, no tumour).
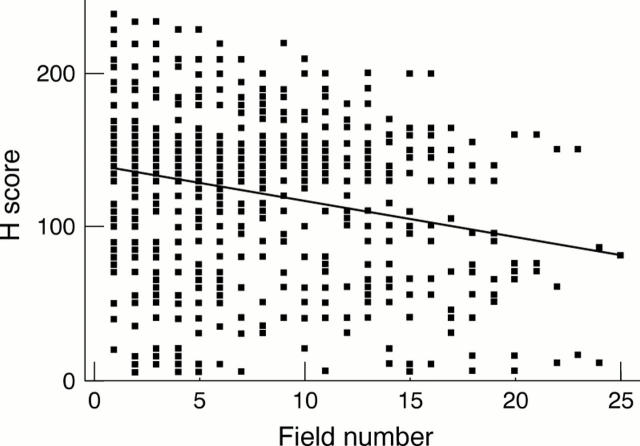
Figure 2 Plot of all H scores from all excised tumours plotted from tumour edge (field 1) to tumour centre for regression analysis. There is a significant downward trend in oestrogen receptor (ER) positivity as the field number increases (p = 0.001).
Figure 3 Plot of H scores from 10 core biopsy specimens in which the edge could be determined (orientated cores) plotted from tumour edge (field 1) towards tumour centre for regression analysis. For cores there is a no significant downward trend in oestrogen receptor (ER) positivity as the field number increases.
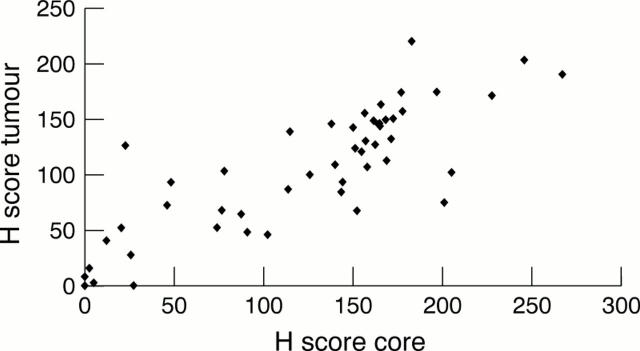
Figure 4 Plot of mean of all H scores from core specimens against mean of all H scores from subsequently excised tumours. There is a significant correlation between oestrogen receptor (ER) positivity in cores and subsequently excised tumours (correlation coefficient = 0.876; p = 0.001).
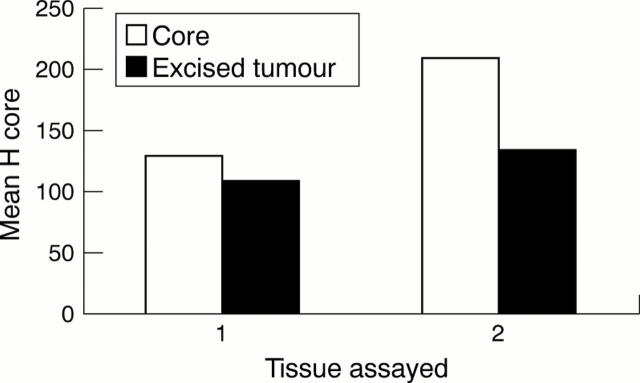
Figure 5 1: Plot of mean of H scores from all core specimens (n = 51) against mean of H scores from all subsequently excised tumours (n = 51) when oestrogen receptor (ER) immunocytochemistry (ICC) was performed on different slides in different assays. 2: Plot of mean of H scores from a sample of core specimens (n = 10) against mean of H scores from subsequently excised tumours (n = 10) when ER ICC was performed on the same slide in one assay. ER positivity is significantly higher in the cores (p = 0.05, paired rank test) using both approaches.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. M., Harris W. H., Smith P., Millis R. R., Rubens R. D. Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer. 1996 Nov;74(9):1445–1451. doi: 10.1038/bjc.1996.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg A., Fernö M., Idvall I. Estrogen receptor enzyme immunoassay in fine-needle aspirates from human breast cancer. Acta Oncol. 1989;28(2):187–191. doi: 10.3109/02841868909111245. [DOI] [PubMed] [Google Scholar]
- Britton P. D., Flower C. D., Freeman A. H., Sinnatamby R., Warren R., Goddard M. J., Wight D. G., Bobrow L. Changing to core biopsy in an NHS breast screening unit. Clin Radiol. 1997 Oct;52(10):764–767. doi: 10.1016/s0009-9260(97)80156-0. [DOI] [PubMed] [Google Scholar]
- Davis B. W., Zava D. T., Locher G. W., Goldhirsch A., Hartmann W. H. Receptor heterogeneity of human breast cancer as measured by multiple intratumoral assays of estrogen and progesterone receptor. Eur J Cancer Clin Oncol. 1984 Mar;20(3):375–382. doi: 10.1016/0277-5379(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Hill A., Freedman B., Gore S. M., Roberts M. M., Forrest A. P. Reproducibility of measurements of oestrogen-receptor concentration in breast cancer. Br J Cancer. 1977 Sep;36(3):355–361. doi: 10.1038/bjc.1977.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin H. J., Isola J. J., Helin M. J., Helle M. J., Krohn K. J. Imprint cytology in immunocytochemical analysis of oestrogen and progesterone receptors of breast carcinoma. J Clin Pathol. 1989 Oct;42(10):1043–1045. doi: 10.1136/jcp.42.10.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S. W., Bédard Y. C. Estrogen and progesterone receptor contents in ThinPrep-processed fine-needle aspirates of breast. Am J Clin Pathol. 1999 Jul;112(1):50–56. doi: 10.1093/ajcp/112.1.50. [DOI] [PubMed] [Google Scholar]
- Makris A., Allred D. C., Powles T. J., Dowsett M., Fernando I. N., Trott P. A., Ashley S. E., Ormerod M. G., Titley J. C., Osborne C. K. Cytological evaluation of biological prognostic markers from primary breast carcinomas. Breast Cancer Res Treat. 1997 May;44(1):65–74. doi: 10.1023/a:1005717924761. [DOI] [PubMed] [Google Scholar]
- Marrazzo A., Taormina P., Leonardi P., Lupo F., Filosto S. Immunocytochemical determination of estrogen and progesterone receptors on 219 fine-needle aspirates of breast cancer. A prospective study. Anticancer Res. 1995 Mar-Apr;15(2):521–526. [PubMed] [Google Scholar]
- McCarty K. S., Jr, Miller L. S., Cox E. B., Konrath J., McCarty K. S., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985 Aug;109(8):716–721. [PubMed] [Google Scholar]
- Nizzoli R., Bozzetti C., Naldi N., Guazzi A., Gabrielli M., Michiara M., Camisa R., Barilli A., Cocconi G. Comparison of the results of immunocytochemical assays for biologic variables on preoperative fine-needle aspirates and on surgical specimens of primary breast carcinomas. Cancer. 2000 Feb 25;90(1):61–66. [PubMed] [Google Scholar]
- Ozzello L., DeRosa C., Habif D. V., Greene G. L. An immunohistochemical evaluation of progesterone receptor in frozen sections, paraffin sections, and cytologic imprints of breast carcinomas. Cancer. 1991 Jan 15;67(2):455–462. doi: 10.1002/1097-0142(19910115)67:2<455::aid-cncr2820670223>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Paridaens R., Sylvester R. J., Ferrazzi E., Legros N., Leclercq G., Heuson J. C. Clinical significance of the quantitative assessment of estrogen receptors in advanced breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2889–2895. doi: 10.1002/1097-0142(19801215)46:12+<2889::aid-cncr2820461430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rhodes A., Jasani B., Barnes D. M., Bobrow L. G., Miller K. D. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000 Feb;53(2):125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silfverswärd C., Skoog L., Humla S., Gustafsson S. A., Nordenskjöld B. Intratumoral variation of cytoplasmic and nuclear estrogen receptor concentrations in human mammary carcinoma. Eur J Cancer. 1980 Jan;16(1):59–65. doi: 10.1016/0014-2964(80)90108-5. [DOI] [PubMed] [Google Scholar]
- van Netten J. P., Algard F. T., Coy P., Carlyle S. J., Brigden M. L., Thornton K. R., To M. P. Estrogen receptor assay on breast cancer microsamples: implications of percent carcinoma estimation. Cancer. 1982 Jun 1;49(11):2383–2388. doi: 10.1002/1097-0142(19820601)49:11<2383::aid-cncr2820491127>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]