Abstract
Aims—Viral uveitis and retinitis, usually caused by herpesviruses, are common in immunosuppressed patients. The diagnosis of viral anterior uveitis and retinitis is usually clinical. The polymerase chain reaction (PCR) has been used for the diagnosis of some viral infections, especially those caused by herpesviruses. This paper reports the use of PCR in the diagnosis of viral retinitis in vitreous samples from Brazilian patients.
Methods—PCR was used for the diagnosis of necrotising retinitis in vitreous samples from patients from the Hospital São Geraldo, Universidade Federal de Minas Gerais, Brazil. The vitreous samples were collected by paracentesis and stored until analysis. Samples were analysed by PCR using specific primers designed to amplify herpes simplex virus 1 (HSV-1), varicella zoster virus (VZV), or human cytomegalovirus (HCMV). In a case of anterior uveitis, PCR was performed with a sample from the anterior chamber.
Results—Herpesvirus DNA was amplified in 11 of 17 samples. HCVM DNA was detected in nine samples but DNA from HSV-1 and VZV were detected only once each.
Conclusion—These results strongly suggest that PCR could be used for a rapid complementary diagnosis of viral uveitis and retinitis. A prospective study to evaluate the PCR results, clinical evolution, and treatment is imperative to corroborate the real value of PCR in diagnosis and how it could help the clinicians' approach.
Key Words: polymerase chain reaction • uveitis • retinitis
Full Text
The Full Text of this article is available as a PDF (117.7 KB).
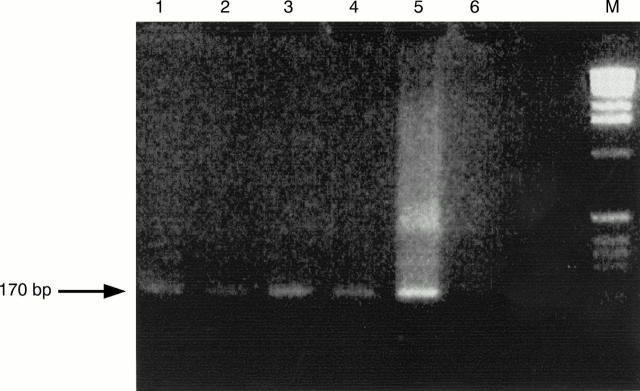
Figure 1 PCR amplification of four samples. The samples were treated with proteinase K, amplified by PCR using human cytomegalovirus (CMV) specific primers, electrophoresed in 2% agarose gel, stained with ethidium bromide, and visualised under UV light (320 nm). Lanes 1–4, positive samples; lane 5, positive control (strain AD169 of human CMV); lane 6, negative control; and lane M, molecular weight marker. The arrow shows a 170 bp fragment.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Tsuchida K., Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996 Jul;234(7):419–424. doi: 10.1007/BF02539407. [DOI] [PubMed] [Google Scholar]
- Boivin G., Handfield J., Toma E., Murray G., Lalonde R., Tevere V. J., Sun R., Bergeron M. G. Evaluation of the AMPLICOR cytomegalovirus test with specimens from human immunodeficiency virus-infected subjects. J Clin Microbiol. 1998 Sep;36(9):2509–2513. doi: 10.1128/jcm.36.9.2509-2513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. H., Glasgow B. J., Holland G. N., Foos R. Y. Cytomegalovirus infection of the conjunctiva in AIDS. Am J Ophthalmol. 1988 Jul 15;106(1):102–104. doi: 10.1016/s0002-9394(14)76402-5. [DOI] [PubMed] [Google Scholar]
- Danise A., Cinque P., Vergani S., Candino M., Racca S., De Bona A., Novati R., Castagna A., Lazzarin A. Use of polymerase chain reaction assays of aqueous humor in the differential diagnosis of retinitis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1997 Jun;24(6):1100–1106. doi: 10.1086/513625. [DOI] [PubMed] [Google Scholar]
- Dennett C., Cleator G. M., Klapper P. E. HSV-1 and HSV-2 in herpes simplex encephalitis: a study of sixty-four cases in the United Kingdom. J Med Virol. 1997 Sep;53(1):1–3. doi: 10.1002/(sici)1096-9071(199709)53:1<1::aid-jmv1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Field H. J., Goldthorpe S. E. The pathogenicity of drug-resistant variants of herpes simplex virus. Res Virol. 1992 Mar-Apr;143(2):120–124. doi: 10.1016/s0923-2516(06)80092-0. [DOI] [PubMed] [Google Scholar]
- Freeman W. R., Lerner C. W., Mines J. A., Lash R. S., Nadel A. J., Starr M. B., Tapper M. L. A prospective study of the ophthalmologic findings in the acquired immune deficiency syndrome. Am J Ophthalmol. 1984 Feb;97(2):133–142. doi: 10.1016/s0002-9394(14)76082-9. [DOI] [PubMed] [Google Scholar]
- Garweg J., Fenner T., Böhnke M., Schmitz H. An improved technique for the diagnosis of viral retinitis from samples of aqueous humor and vitreous. Graefes Arch Clin Exp Ophthalmol. 1993 Sep;231(9):508–513. doi: 10.1007/BF00921115. [DOI] [PubMed] [Google Scholar]
- Holland G. N., Pepose J. S., Pettit T. H., Gottlieb M. S., Yee R. D., Foos R. Y. Acquired immune deficiency syndrome. Ocular manifestations. Ophthalmology. 1983 Aug;90(8):859–873. doi: 10.1016/s0161-6420(83)80009-8. [DOI] [PubMed] [Google Scholar]
- Holland G. N. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994 May 15;117(5):663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- Holland G. N. The progressive outer retinal necrosis syndrome. Int Ophthalmol. 1994;18(3):163–165. doi: 10.1007/BF00915966. [DOI] [PubMed] [Google Scholar]
- Mitchell S. M., Fox J. D., Tedder R. S., Gazzard B. G., Lightman S. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994 Aug;43(4):336–340. doi: 10.1002/jmv.1890430404. [DOI] [PubMed] [Google Scholar]
- Muccioli C., Belfort Júnior R., Lottenberg C., Lima J., Santos P., Kim M., de Abreu M. T., Neves R. Achados oftalmológicos em AIDS: avaliaço de 445 casos atendidos em um ano. Rev Assoc Med Bras (1992) 1994 Jul-Sep;40(3):155–158. [PubMed] [Google Scholar]
- Nahass G. T., Goldstein B. A., Zhu W. Y., Serfling U., Penneys N. S., Leonardi C. L. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992 Nov 11;268(18):2541–2544. [PubMed] [Google Scholar]
- Nogueira M. L., Carvalho A. F., Barbosa E. F., Bonjardim C. A., Ferreira P. C., Kroon E. G. Diagnosis of mucocutaneous herpetic infections by PCR without DNA extraction. Mem Inst Oswaldo Cruz. 1998 Mar-Apr;93(2):213–214. doi: 10.1590/s0074-02761998000200015. [DOI] [PubMed] [Google Scholar]
- O'Brien J. J., Campoli-Richards D. M. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989 Mar;37(3):233–309. doi: 10.2165/00003495-198937030-00002. [DOI] [PubMed] [Google Scholar]
- Schacher S., Garweg J. G., Russ C., Böhnke M. Die Diagnostik der herpetischen Uveitis und Keratouveitis. Klin Monbl Augenheilkd. 1998 May;212(5):359–362. doi: 10.1055/s-2008-1034906. [DOI] [PubMed] [Google Scholar]
- Short G. A., Margolis T. P., Kuppermann B. D., Irvine A. R., Martin D. F., Chandler D. A polymerase chain reaction-based assay for diagnosing varicella-zoster virus retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1997 Feb;123(2):157–164. doi: 10.1016/s0002-9394(14)71031-1. [DOI] [PubMed] [Google Scholar]
- Verbraak F. D., Galema M., van den Horn G. H., Bruinenberg M., Luyendijk L., Danner S. A., Kijlstra A. Serological and polymerase chain reaction-based analysis of aqueous humour samples in patients with AIDS and necrotizing retinitis. AIDS. 1996 Sep;10(10):1091–1099. [PubMed] [Google Scholar]
- Wiedbrauk D. L., Werner J. C., Drevon A. M. Inhibition of PCR by aqueous and vitreous fluids. J Clin Microbiol. 1995 Oct;33(10):2643–2646. doi: 10.1128/jcm.33.10.2643-2646.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Pavan-Langston D., Kinoshita S., Nishida K., Shimomura Y., Tano Y. Detecting herpesvirus DNA in uveitis using the polymerase chain reaction. Br J Ophthalmol. 1996 May;80(5):465–468. doi: 10.1136/bjo.80.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. H., Verhagen C., Bruinenberg M., Rothova A., de Jong P. T., Baarsma G. S., Van der Lelij A., Ooyman F. M., Bollemeijer J. G., Derhaag P. J. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996 Jun;121(6):650–658. doi: 10.1016/s0002-9394(14)70631-2. [DOI] [PubMed] [Google Scholar]