Abstract
Background/Aims—The pathogenesis of idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP), a chronic and incurable human respiratory disease, is not well established. This study was designed to investigate whether the apoptosis of type II pneumocytes could be the precipitating factor in the pathogenesis of IPF.
Methods—Nineteen specimens obtained by retrospective review of the medical and pathological records of 55 patients with IPF, four normal subjects, and 10 disease control lungs were analysed. The selected specimens had normal alveoli with intervening patchy scarring of the lung parenchyma, fulfilling the pathological criteria for UIP. To identify individual cells undergoing apoptosis in the normal alveoli, electron microscopy and in situ end labelling of fragmented DNA were performed on paraffin wax embedded sections using digoxigenin-11-dUTP and the enzyme terminal deoxynucleotidyl transferase.
Results—Apoptosis was detected in the normal alveoli of 17 of the 19 patients with IPF/UIP and was absent in the controls. Electron microscopy demonstrated apoptotic changes in type II pneumocytes. These results indicate that apoptotic type II pneumocyte death occurs in normal alveoli of IPF/UIP and could be the principal cause of several events that account for the histological, clinical, and functional alterations seen in IPF/UIP.
Conclusions—In conclusion, numerous type II pneumocytes from the normal alveoli of most patients with IPF/UIP actively undergo programmed cell death. This finding may shed new light on the pathogenesis of this disease, with implications mainly for the treatment of affected patients.
Key Words: apoptosis • type II pneumocytes • idiopathic pulmonary fibrosis • pathogenesis
Full Text
The Full Text of this article is available as a PDF (239.9 KB).
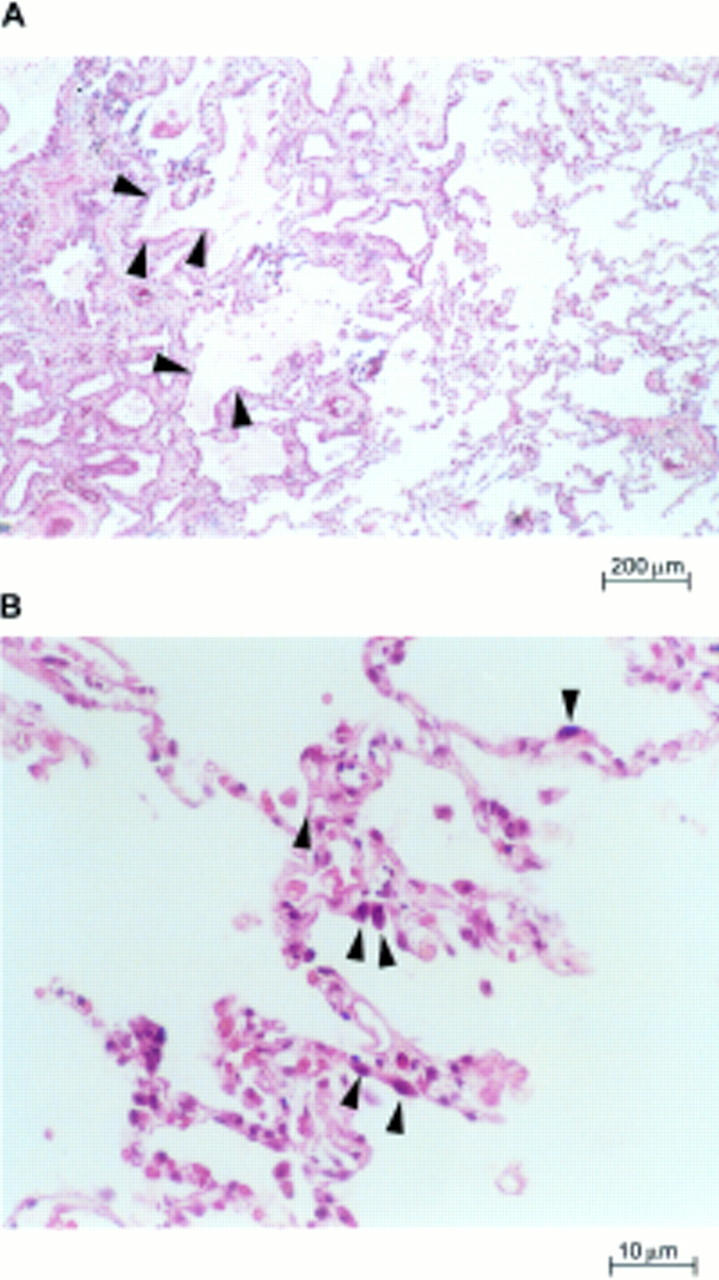
Figure 1 Lung parenchyma of patients with usual interstitial pneumonia. (A) Characteristic variation in histological appearance from one area to another. Dense collagen deposition in the lung parenchyma is seen on the left, and patchy areas containing normal or nearly normal alveoli are present nearby (right and bottom centre). There is a zone of microscopic honeycomb change at the middle left (arrowheads) that is characterised by mucin filled, enlarged air spaces separated by fibrosis (haematoxylin and eosin staining; magnification, x100). (B) High magnification view of the surviving zones of alveoli showing marginated masses of chromatin within shaped, rounded alveolar cells with eosinophilic cytoplasm (arrowheads), suggestive of type II pneumocytes (haematoxylin and eosin staining; magnification, x400).
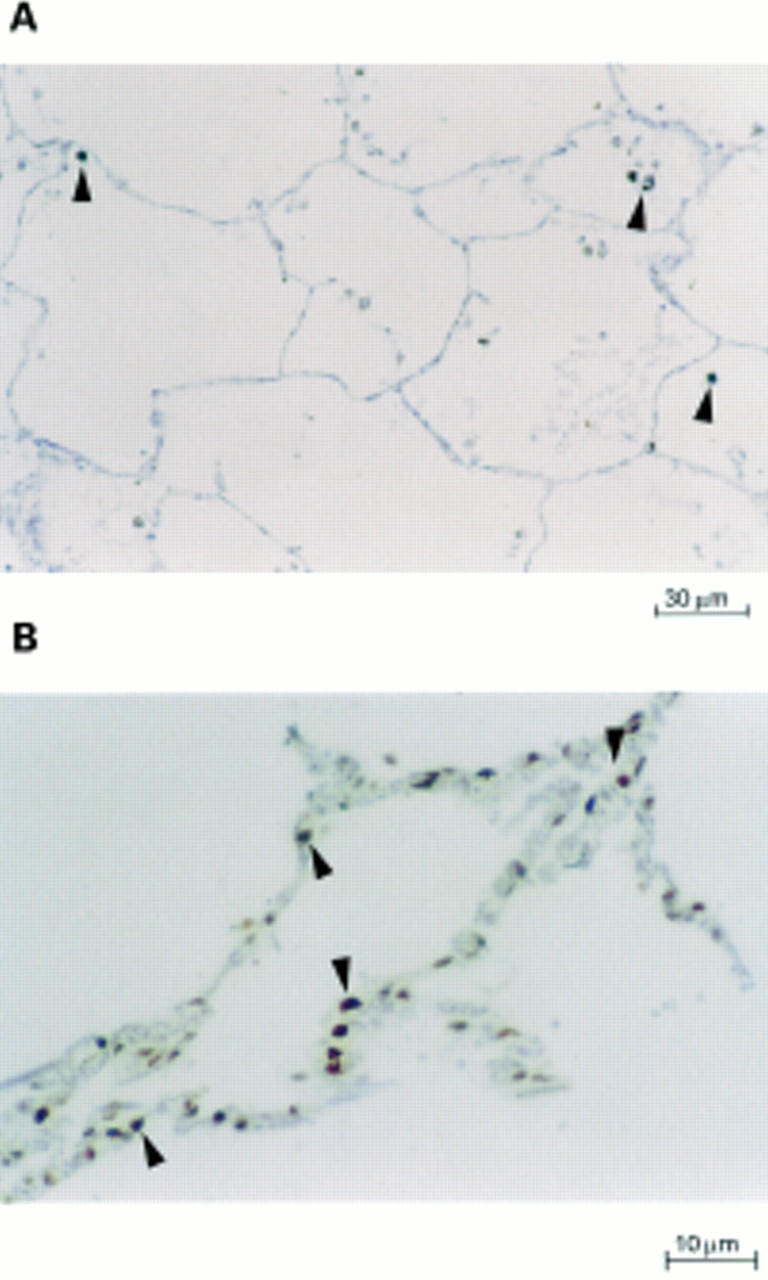
Figure 2 In situ end labelling of fragmented DNA with terminal deoxynucleotidyl transferase and digoxigenin-11-dUTP. Cells with fragmented DNA stained brown, whereas cells with normal nuclei stained blue (immunoperoxidase staining with haematoxylin counterstaining). (A) A section from a normal lung shows no apoptotic nuclei (magnification, x160). The intra-alveolar cells seen are macrophages containing carbon pigment (arrowheads). (B) Lung sections from patients with usual interstitial pneumonia (magnification, x400) with increased labelling. Numerous alveolar nuclei undergoing apoptosis suggestive of type II pneumocytes (arrowheads).
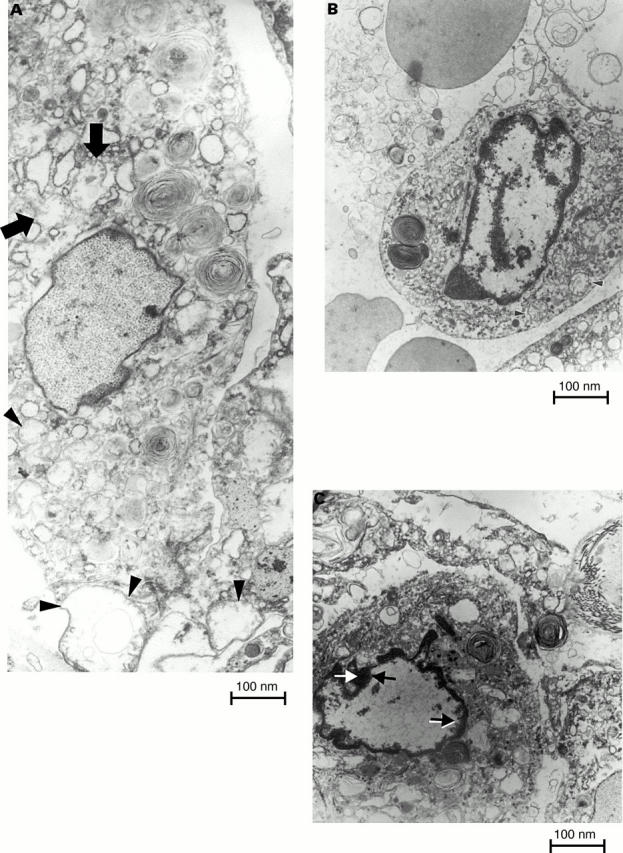
Figure 3 Sections of lung from patients with usual interstitial pneumonia examined by electron microscopy. (A) Type II pneumocytes undergoing non-explicit apoptosis, characterised by fine and uniformly dispersed chromatin within the nuclear membrane. These pneumocytes have swollen mitochondrial profiles, sometimes with rupture of the outer membrane because of the pressure of the inner mitochondrial membrane, which surrounds a greatly swollen inner mitochondrial compartment (arrows and arrows heads). (B and C) Type II pneumocytes with very different nuclear profiles to those of non-apoptotic cells, characterised by progressive condensation of the chromatin as part of a clarifying process of the remaining nuclear region and a final degradation of the cytoplasmic structure (magnification, x9000).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Adamson I. Y., Young L., Bowden D. H. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988 Feb;130(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- Angermüller S., Künstle G., Tiegs G. Pre-apoptotic alterations in hepatocytes of TNFalpha-treated galactosamine-sensitized mice. J Histochem Cytochem. 1998 Oct;46(10):1175–1183. doi: 10.1177/002215549804601009. [DOI] [PubMed] [Google Scholar]
- Basset F., Ferrans V. J., Soler P., Takemura T., Fukuda Y., Crystal R. G. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986 Mar;122(3):443–461. [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am Rev Respir Dis. 1989 Aug;140(2):513–524. doi: 10.1164/ajrccm/140.2.513. [DOI] [PubMed] [Google Scholar]
- Carrington C. B., Gaensler E. A., Coutu R. E., FitzGerald M. X., Gupta R. G. Natural history and treated course of usual and desquamative interstitial pneumonia. N Engl J Med. 1978 Apr 13;298(15):801–809. doi: 10.1056/NEJM197804132981501. [DOI] [PubMed] [Google Scholar]
- Chauncey J. B., Peters-Golden M., Simon R. H. Arachidonic acid metabolism by rat alveolar epithelial cells. Lab Invest. 1988 Feb;58(2):133–140. [PubMed] [Google Scholar]
- Cherniack R. M., Crystal R. G., Kalica A. R. NHLBI Workshop summary. Current concepts in idiopathic pulmonary fibrosis: a road map for the future. Am Rev Respir Dis. 1991 Mar;143(3):680–683. doi: 10.1164/ajrccm/143.3.680. [DOI] [PubMed] [Google Scholar]
- Coxson H. O., Hogg J. C., Mayo J. R., Behzad H., Whittall K. P., Schwartz D. A., Hartley P. G., Galvin J. R., Wilson J. S., Hunninghake G. W. Quantification of idiopathic pulmonary fibrosis using computed tomography and histology. Am J Respir Crit Care Med. 1997 May;155(5):1649–1656. doi: 10.1164/ajrccm.155.5.9154871. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Gehr P., Bachofen M., Weibel E. R. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982 Aug;126(2):332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Bienkowski R. S., Cowan M. J., Breul S. D., Bradley K. M., Ferrans V. J., Roberts W. C., Crystal R. G. Collagen concentration and rates of synthesis in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1980 Aug;122(2):289–301. doi: 10.1164/arrd.1980.122.2.289. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. H., Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem. 1982 Aug 10;257(15):8630–8633. [PubMed] [Google Scholar]
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998 Aug 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hartman T. E., Primack S. L., Kang E. Y., Swensen S. J., Hansell D. M., McGuinness G., Müller N. L. Disease progression in usual interstitial pneumonia compared with desquamative interstitial pneumonia. Assessment with serial CT. Chest. 1996 Aug;110(2):378–382. doi: 10.1378/chest.110.2.378. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Kalica A. R. Approaches to the treatment of pulmonary fibrosis. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 1):915–918. doi: 10.1164/ajrccm.151.3.7881692. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Fiorelli R. F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994 Feb;18(2):136–147. [PubMed] [Google Scholar]
- Katzenstein A. L., Myers J. L. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L., Myers J. L., Mazur M. T. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986 Apr;10(4):256–267. [PubMed] [Google Scholar]
- Katzenstein A. L. Pathogenesis of "fibrosis" in interstitial pneumonia: an electron microscopic study. Hum Pathol. 1985 Oct;16(10):1015–1024. doi: 10.1016/s0046-8177(85)80279-3. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982 Jan;46(1):39–53. [PubMed] [Google Scholar]
- Kazufumi M., Sonoko N., Masanori K., Takateru I., Akira O. Expression of bcl-2 protein and APO-1 (Fas antigen) in the lung tissue from patients with idiopathic pulmonary fibrosis. Microsc Res Tech. 1997 Sep 1;38(5):480–487. doi: 10.1002/(SICI)1097-0029(19970901)38:5<480::AID-JEMT4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K., Miyazaki H., Hagimoto N., Kawasaki M., Fujita M., Kunitake R., Kaneko Y., Hara N. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol. 1999 Jan;20(1):53–60. doi: 10.1165/ajrcmb.20.1.2941. [DOI] [PubMed] [Google Scholar]
- McCormack F. X., King T. E., Jr, Bucher B. L., Nielsen L., Mason R. J., McCormac F. X. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995 Aug;152(2):751–759. doi: 10.1164/ajrccm.152.2.7633738. [DOI] [PubMed] [Google Scholar]
- Mol H. G., van Dam R. C., Vreeken R. J., Steijger O. M. Determination of daminozide in apples and apple leaves by liquid chromatography--mass spectrometry. J Chromatogr A. 1999 Feb 12;833(1):53–60. doi: 10.1016/s0021-9673(98)00915-7. [DOI] [PubMed] [Google Scholar]
- Myers J. L., Katzenstein A. L. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest. 1988 Dec;94(6):1309–1311. doi: 10.1378/chest.94.6.1309. [DOI] [PubMed] [Google Scholar]
- Robinson P. C., Watters L. C., King T. E., Mason R. J. Idiopathic pulmonary fibrosis. Abnormalities in bronchoalveolar lavage fluid phospholipids. Am Rev Respir Dis. 1988 Mar;137(3):585–591. doi: 10.1164/ajrccm/137.3.585. [DOI] [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L., Polgar P., McAteer J. A., Douglas W. H. Prostaglandin production by type II alveolar epithelial cells. Biochim Biophys Acta. 1979 Mar 29;572(3):502–509. doi: 10.1016/0005-2760(79)90157-7. [DOI] [PubMed] [Google Scholar]
- Tubbs R. R., Benjamin S. P., Reich N. E., McCormack L. J., Van Ordstrand H. S. Desquamative interstitial pneumonitis. Cellular phase of fibrosing alveolitis. Chest. 1977 Aug;72(2):159–165. doi: 10.1378/chest.72.2.159. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997 Nov 28;91(5):627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wilson T. A., Bachofen H. A model for mechanical structure of the alveolar duct. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):1064–1070. doi: 10.1152/jappl.1982.52.4.1064. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]


