Abstract
Cytomegalovirus (CMV) is a recognised cause of morbidity and mortality in immunocompromised individuals. This review will concentrate on recent advances in the understanding of the complex interplay between the host and parasite and the pathological consequences of perturbation of the host immune system. The classic view of CMV as a slowly replicating virus is challenged by recent in vivo findings suggesting that active replication occurs dynamically in the human host, with a doubling time of approximately one day. In addition, CMV load plays a major role in viral pathogenesis, such that increased CMV replication is a significant risk factor for disease in all immunocompromised groups studied to date. These studies focus attention on understanding the virological and immunological determinants of enhanced viral replication and its pathological consequences.
Key Words: polymerase chain reaction • quantitation • inclusion bodies • antiviral chemotherapy
Full Text
The Full Text of this article is available as a PDF (129.6 KB).
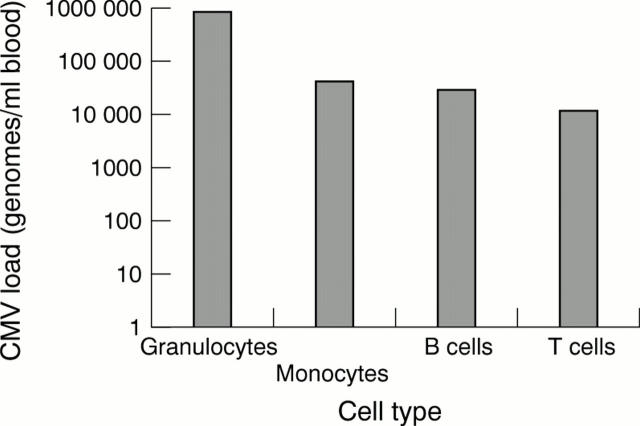
Figure 1 Distribution of cytomegalovirus (CMV) load in cell types present in the blood (granulocytes/neutrophils, monocytes, B cells, and T cells) during an active CMV infection of a renal transplant recipient. Cell subsets were purified using Dynabeads coated with the appropriate antibody and a combination of positive and negative selection according to the recommendations of the manufacturer.
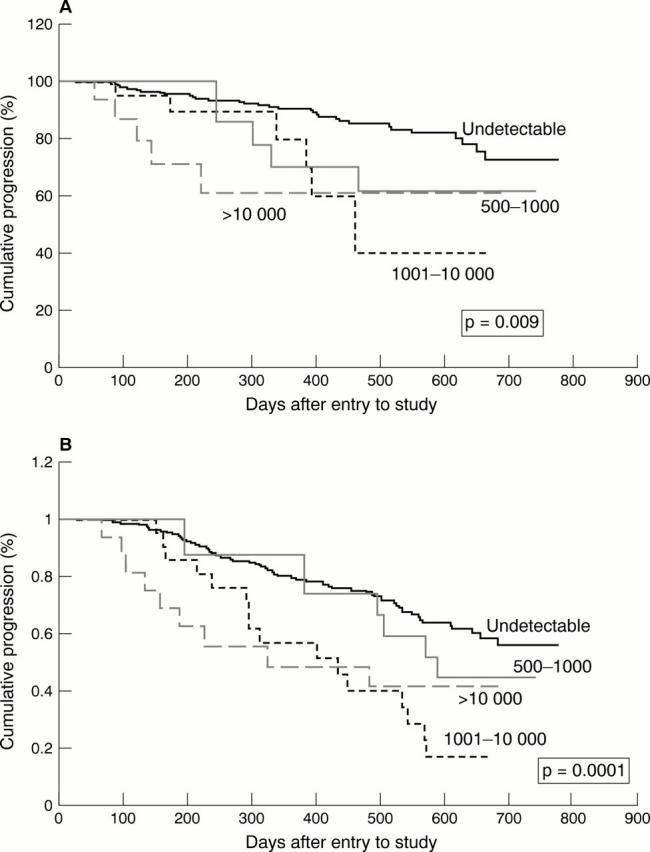
Figure 2 Kaplan-Meier survival curves showing the influence of baseline cytomegalovirus (CMV) load in the blood on (A) time to disease and (B) death in a cohort of human immunodeficiency virus (HIV) infected individuals enrolled in ACTG204.58
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodaghi B., Jones T. R., Zipeto D., Vita C., Sun L., Laurent L., Arenzana-Seisdedos F., Virelizier J. L., Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998 Sep 7;188(5):855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego F., Ulbrecht M., Weiss E. H., Coligan J. E., Brooks A. G. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998 Mar 2;187(5):813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen E. F., Emery V. C., Wilson P., Johnson M. A., Davey C. C., Sabin C. A., Farmer D., Griffiths P. D. Cytomegalovirus polymerase chain reaction viraemia in patients receiving ganciclovir maintenance therapy for retinitis. AIDS. 1998 Apr 16;12(6):605–611. doi: 10.1097/00002030-199806000-00009. [DOI] [PubMed] [Google Scholar]
- Bowen E. F., Sabin C. A., Wilson P., Griffiths P. D., Davey C. C., Johnson M. A., Emery V. C. Cytomegalovirus (CMV) viraemia detected by polymerase chain reaction identifies a group of HIV-positive patients at high risk of CMV disease. AIDS. 1997 Jun;11(7):889–893. doi: 10.1097/00002030-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Bowen E. F., Wilson P., Cope A., Sabin C., Griffiths P., Davey C., Johnson M., Emery V. Cytomegalovirus retinitis in AIDS patients: influence of cytomegaloviral load on response to ganciclovir, time to recurrence and survival. AIDS. 1996 Nov;10(13):1515–1520. doi: 10.1097/00002030-199611000-00009. [DOI] [PubMed] [Google Scholar]
- Browne H., Churcher M., Minson T. Construction and characterization of a human cytomegalovirus mutant with the UL18 (class I homolog) gene deleted. J Virol. 1992 Nov;66(11):6784–6787. doi: 10.1128/jvi.66.11.6784-6787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne H., Smith G., Beck S., Minson T. A complex between the MHC class I homologue encoded by human cytomegalovirus and beta 2 microglobulin. Nature. 1990 Oct 25;347(6295):770–772. doi: 10.1038/347770a0. [DOI] [PubMed] [Google Scholar]
- Cha T. A., Tom E., Kemble G. W., Duke G. M., Mocarski E. S., Spaete R. R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996 Jan;70(1):78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Chou S. W., Dennison K. M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991 Jun;163(6):1229–1234. doi: 10.1093/infdis/163.6.1229. [DOI] [PubMed] [Google Scholar]
- Chou S. W. Reactivation and recombination of multiple cytomegalovirus strains from individual organ donors. J Infect Dis. 1989 Jul;160(1):11–15. doi: 10.1093/infdis/160.1.11. [DOI] [PubMed] [Google Scholar]
- Chou S. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology. 1992 May;188(1):388–390. doi: 10.1016/0042-6822(92)90771-g. [DOI] [PubMed] [Google Scholar]
- Cope A. V., Sabin C., Burroughs A., Rolles K., Griffiths P. D., Emery V. C. Interrelationships among quantity of human cytomegalovirus (HCMV) DNA in blood, donor-recipient serostatus, and administration of methylprednisolone as risk factors for HCMV disease following liver transplantation. J Infect Dis. 1997 Dec;176(6):1484–1490. doi: 10.1086/514145. [DOI] [PubMed] [Google Scholar]
- Cope A. V., Sweny P., Sabin C., Rees L., Griffiths P. D., Emery V. C. Quantity of cytomegalovirus viruria is a major risk factor for cytomegalovirus disease after renal transplantation. J Med Virol. 1997 Jun;52(2):200–205. [PubMed] [Google Scholar]
- Emery V. C., Cope A. V., Bowen E. F., Gor D., Griffiths P. D. The dynamics of human cytomegalovirus replication in vivo. J Exp Med. 1999 Jul 19;190(2):177–182. doi: 10.1084/jem.190.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery V. C. Cytomegalovirus drug resistance. Antivir Ther. 1998;3(4):239–242. [PubMed] [Google Scholar]
- Emery V. C., Sabin C. A., Cope A. V., Gor D., Hassan-Walker A. F., Griffiths P. D. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000 Jun 10;355(9220):2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- Emery V. C., Sabin C., Feinberg J. E., Grywacz M., Knight S., Griffiths P. D. Quantitative effects of valacyclovir on the replication of cytomegalovirus (CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. The AIDS Clinical Trials Group 204/Glaxo Wellcome 123-014 International CMV Prophylaxis Study Group. J Infect Dis. 1999 Sep;180(3):695–701. doi: 10.1086/314936. [DOI] [PubMed] [Google Scholar]
- Emery V. C. Tuning to the right frequency: cytotoxic T lymphocytes and cytomegalovirus. Transplantation. 2000 Jun 15;69(11):2241–2242. doi: 10.1097/00007890-200006150-00004. [DOI] [PubMed] [Google Scholar]
- Engstrand M., Tournay C., Peyrat M. A., Eriksson B. M., Wadström J., Wirgart B. Z., Romagné F., Bonneville M., Tötterman T. H., Korsgren O. Characterization of CMVpp65-specific CD8+ T lymphocytes using MHC tetramers in kidney transplant patients and healthy participants. Transplantation. 2000 Jun 15;69(11):2243–2250. doi: 10.1097/00007890-200006150-00005. [DOI] [PubMed] [Google Scholar]
- Fox J. C., Kidd I. M., Griffiths P. D., Sweny P., Emery V. C. Longitudinal analysis of cytomegalovirus load in renal transplant recipients using a quantitative polymerase chain reaction: correlation with disease. J Gen Virol. 1995 Feb;76(Pt 2):309–319. doi: 10.1099/0022-1317-76-2-309. [DOI] [PubMed] [Google Scholar]
- Gilbert M. J., Riddell S. R., Plachter B., Greenberg P. D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996 Oct 24;383(6602):720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- Gor D., Sabin C., Prentice H. G., Vyas N., Man S., Griffiths P. D., Emery V. C. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998 Mar;21(6):597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- Griffiths P. D., Feinberg J. E., Fry J., Sabin C., Dix L., Gor D., Ansari A., Emery V. C. The effect of valaciclovir on cytomegalovirus viremia and viruria detected by polymerase chain reaction in patients with advanced human immunodeficiency virus disease. AIDS Clinical Trials Group Protocol 204/Glaxo Wellcome 123-014 International CMV Prophylaxis Study Group. J Infect Dis. 1998 Jan;177(1):57–64. doi: 10.1086/513806. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Shanley J. D., Griffiths P. D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987 Oct 31;2(8566):996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- Hassan-Walker A. F., Cope A. V., Griffiths P. D., Emery V. C. Transcription of the human cytomegalovirus natural killer decoy gene, UL18, in vitro and in vivo. J Gen Virol. 1998 Sep;79(Pt 9):2113–2116. doi: 10.1099/0022-1317-79-9-2113. [DOI] [PubMed] [Google Scholar]
- Hassan-Walker A. F., Kidd I. M., Sabin C., Sweny P., Griffiths P. D., Emery V. C. Quantity of human cytomegalovirus (CMV) DNAemia as a risk factor for CMV disease in renal allograft recipients: relationship with donor/recipient CMV serostatus, receipt of augmented methylprednisolone and antithymocyte globulin (ATG). J Med Virol. 1999 Jun;58(2):182–187. [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Karavellas M. P., Lowder C. Y., Macdonald C., Avila C. P., Jr, Freeman W. R. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998 Feb;116(2):169–175. doi: 10.1001/archopht.116.2.169. [DOI] [PubMed] [Google Scholar]
- Karavellas M. P., Plummer D. J., Macdonald J. C., Torriani F. J., Shufelt C. L., Azen S. P., Freeman W. R. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999 Mar;179(3):697–700. doi: 10.1086/314639. [DOI] [PubMed] [Google Scholar]
- Kühn J. E., Wendland T., Schäfer P., Möhring K., Wieland U., Elgas M., Eggers H. J. Monitoring of renal allograft recipients by quantitation of human cytomegalovirus genomes in peripheral blood leukocytes. J Med Virol. 1994 Dec;44(4):398–405. doi: 10.1002/jmv.1890440416. [DOI] [PubMed] [Google Scholar]
- Lau J. Y., Davis G. L., Kniffen J., Qian K. P., Urdea M. S., Chan C. S., Mizokami M., Neuwald P. D., Wilber J. C. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993 Jun 12;341(8859):1501–1504. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- Mattes F. M., McLaughlin J. E., Emery V. C., Clark D. A., Griffiths P. D. Histopathological detection of owl's eye inclusions is still specific for cytomegalovirus in the era of human herpesviruses 6 and 7. J Clin Pathol. 2000 Aug;53(8):612–614. doi: 10.1136/jcp.53.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Pande H., Forman S. J., Tanamachi B., Li C. R., Zaia J. A., Greenberg P. D., Riddell S. R. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994 May;43(1):103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Kingsley L. A., Rinaldo C. R., Jr, Todd J. A., Hoo B. S., Kokka R. P., Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995 Apr 15;122(8):573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Millar A. B., Patou G., Miller R. F., Grundy J. E., Katz D. R., Weller I. V., Semple S. J. Cytomegalovirus in the lungs of patients with AIDS. Respiratory pathogen or passenger? Am Rev Respir Dis. 1990 Jun;141(6):1474–1477. doi: 10.1164/ajrccm/141.6.1474. [DOI] [PubMed] [Google Scholar]
- Neumann A. U., Lam N. P., Dahari H., Gretch D. R., Wiley T. E., Layden T. J., Perelson A. S. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998 Oct 2;282(5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- Nowak M. A., Bonhoeffer S., Hill A. M., Boehme R., Thomas H. C., McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlin M., Sundqvist V. A., Mach M., Wahren B., Borrebaeck C. A. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J Virol. 1993 Feb;67(2):703–710. doi: 10.1128/jvi.67.2.703-710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold M. E., Dairaghi D. J., Duke G. M., Saederup N., Mocarski E. S., Kemble G. W., Schall T. J. Cytomegalovirus encodes a potent alpha chemokine. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9839–9844. doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleskoff O., Tréboute C., Brelot A., Heveker N., Seman M., Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997 Jun 20;276(5320):1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L. Viral strategies of immune evasion. Science. 1998 Apr 10;280(5361):248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- Reyburn H. T., Mandelboim O., Valés-Gómez M., Davis D. M., Pazmany L., Strominger J. L. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature. 1997 Apr 3;386(6624):514–517. doi: 10.1038/386514a0. [DOI] [PubMed] [Google Scholar]
- Saltzman R. L., Quirk M. R., Jordan M. C. High levels of circulating cytomegalovirus DNA reflect visceral organ disease in viremic immunosuppressed patients other than marrow recipients. J Clin Invest. 1992 Nov;90(5):1832–1838. doi: 10.1172/JCI116059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai M., Bozzette S. A., Powderly W., Frame P., Spector S. A. Utility of urine and leukocyte cultures and plasma DNA polymerase chain reaction for identification of AIDS patients at risk for developing human cytomegalovirus disease. J Infect Dis. 1997 Feb;175(2):302–308. doi: 10.1093/infdis/175.2.302. [DOI] [PubMed] [Google Scholar]
- Singhal S., Shaw J. C., Ainsworth J., Hathaway M., Gillespie G. M., Paris H., Ward K., Pillay D., Moss P. A., Mutimer D. J. Direct visualization and quantitation of cytomegalovirus-specific CD8+ cytotoxic T-lymphocytes in liver transplant patients. Transplantation. 2000 Jun 15;69(11):2251–2259. doi: 10.1097/00007890-200006150-00006. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Hsia K., Crager M., Pilcher M., Cabral S., Stempien M. J. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J Virol. 1999 Aug;73(8):7027–7030. doi: 10.1128/jvi.73.8.7027-7030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Merrill R., Wolf D., Dankner W. M. Detection of human cytomegalovirus in plasma of AIDS patients during acute visceral disease by DNA amplification. J Clin Microbiol. 1992 Sep;30(9):2359–2365. doi: 10.1128/jcm.30.9.2359-2365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Wong R., Hsia K., Pilcher M., Stempien M. J. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998 Jan 15;101(2):497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire S. B., Lipman M. C., Bagdades E. K., Mulvenna P. M., Grundy J. E., Griffiths P. D., Johnson M. A. Severe cytomegalovirus pneumonitis in HIV infected patients with higher than average CD4 counts. Thorax. 1992 Apr;47(4):301–304. doi: 10.1136/thx.47.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S., Reynolds D. W., Tsiantos A., Fuccillo D. A., Long W., Alford C. A. Comparative serial virologic and serologic studies of symptomatic and subclinical congenitally and natally acquired cytomegalovirus infections. J Infect Dis. 1975 Nov;132(5):568–577. doi: 10.1093/infdis/132.5.568. [DOI] [PubMed] [Google Scholar]
- Söderberg-Nauclér C., Fish K. N., Nelson J. A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997 Oct 3;91(1):119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- Wagner B., Kropff B., Kalbacher H., Britt W., Sundqvist V. A., Ostberg L., Mach M. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J Virol. 1992 Sep;66(9):5290–5297. doi: 10.1128/jvi.66.9.5290-5297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Wills M. R., Carmichael A. J., Mynard K., Jin X., Weekes M. P., Plachter B., Sissons J. G. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996 Nov;70(11):7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]