Abstract
Aim—To compare the sensitivity and staining pattern of the new immunohistochemical antibody to tyrosinase (T311) with S-100, HMB45, and the recently evaluated antibody to melan-A (A103) in a range of melanocytic lesions.
Method—Archival, formalin fixed, paraffin wax embedded sections from 50 benign and malignant melanocytic lesions were stained immunohistochemically with anti-tyrosinase, A103, S-100, and HMB45. They were scored semiquantitatively for the distribution and intensity of staining.
Results—All melanomas, with the exception of desmoplastic melanoma, showed some staining with all four antibodies. Overall, T311 and A103 showed an intermediate sensitivity compared with that of S-100 and HMB45. T311 stained most benign and malignant lesions strongly and diffusely with minimal background staining. Immunoreactivity was found to be patchy in some naevi, with weak or absent staining of the mature melanocytes. A103 showed strong and diffuse staining of all benign lesions and most melanomas with minimal background staining. S-100 was the most sensitive, with diffuse staining of most lesions, including desmoplastic and metastatic melanoma, but lacked specificity. HMB45 was the least sensitive antibody, frequently demonstrating patchy staining with absent staining in some benign naevi.
Conclusions—S-100 remains the most sensitive marker of melanocytes. However, because of its lack of specificity, it should be used with at least one other more specific antibody. HMB45 is more specific, but lacks sensitivity; T311 is a reliable marker of melanocytes in paraffin wax embedded sections and is worth consideration for use in a staining panel, although it shows no additional benefit over A103.
Key Words: T311 (anti-tyrosinase) • A103 (anti melan-A) • immunohistochemistry • melanocytic lesions
Full Text
The Full Text of this article is available as a PDF (237.6 KB).
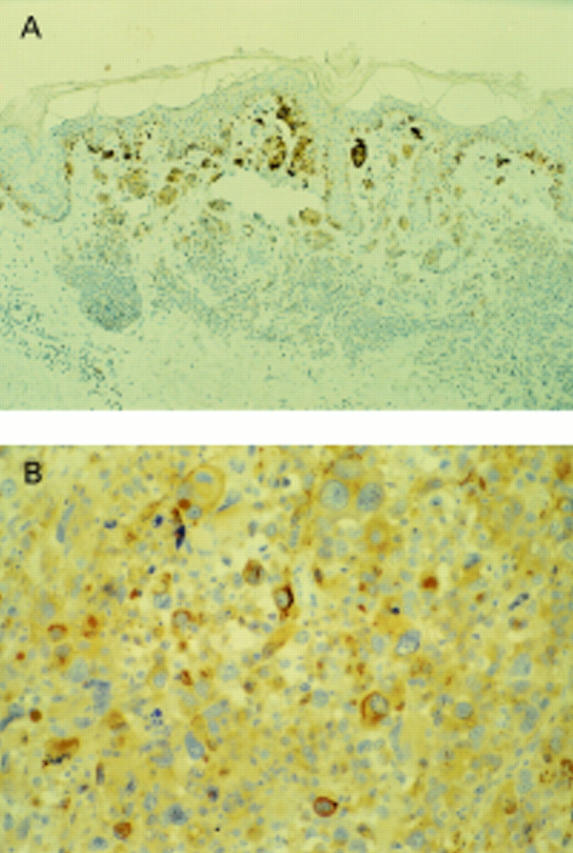
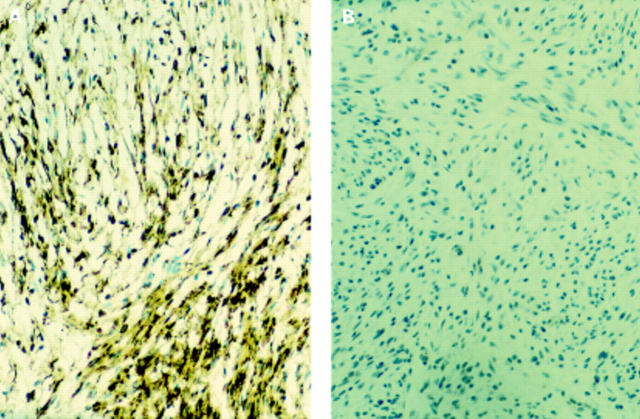
Figure 1 (A) Compound naevus: T311 stains junctional and superficial dermal melanocytes strongly, with weak staining of the mature component. (B) Metastatic melanoma in a lymph node: T311 shows strong patchy staining.
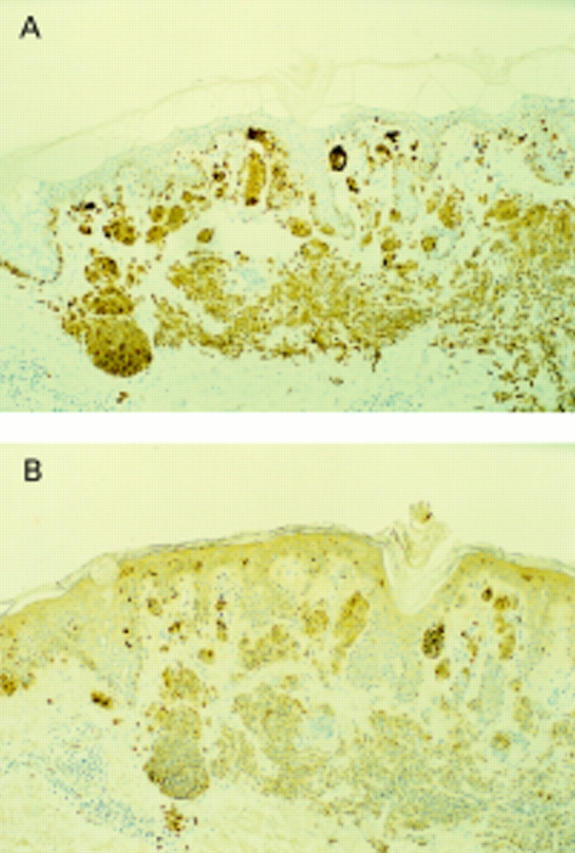
Figure 2 (A) Compound naevus: A103 shows strong diffuse staining of all melanocytes. (B) Compound naevus: S-100 stains melanocytes strongly and diffusely.
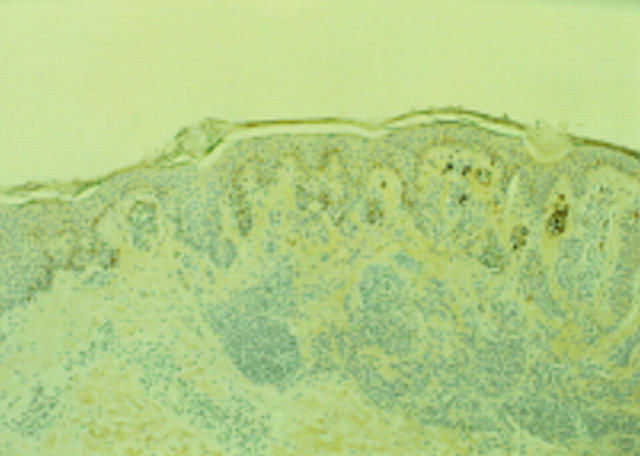
Figure 3 Desmoplastic melanoma. (A) S-100 showing strong diffuse staining. (B) No staining with T311.
Figure 4 Compound naevus. HMB45 showing immunoreactivity of the junctional and superficial dermal melanocytes with no staining of the mature component.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., de Boer A. J., Vogel A. M., Loenen W. A., Figdor C. G. Molecular characterization of the melanocyte lineage-specific antigen gp100. J Biol Chem. 1994 Aug 5;269(31):20126–20133. [PubMed] [Google Scholar]
- Adema G. J., de Boer A. J., van 't Hullenaar R., Denijn M., Ruiter D. J., Vogel A. M., Figdor C. G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993 Dec;143(6):1579–1585. [PMC free article] [PubMed] [Google Scholar]
- Blaheta H. J., Schittek B., Breuninger H., Maczey E., Kroeber S., Sotlar K., Ellwanger U., Thelen M. H., Rassner G., Bültmann B. Lymph node micrometastases of cutaneous melanoma: increased sensitivity of molecular diagnosis in comparison to immunohistochemistry. Int J Cancer. 1998 Aug 21;79(4):318–323. doi: 10.1002/(sici)1097-0215(19980821)79:4<318::aid-ijc3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Blessing K., Sanders D. S., Grant J. J. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998 Feb;32(2):139–146. doi: 10.1046/j.1365-2559.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- Busam K. J., Chen Y. T., Old L. J., Stockert E., Iversen K., Coplan K. A., Rosai J., Barnhill R. L., Jungbluth A. A. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol. 1998 Aug;22(8):976–982. doi: 10.1097/00000478-199808000-00007. [DOI] [PubMed] [Google Scholar]
- Busam K. J., Iversen K., Coplan K. A., Old L. J., Stockert E., Chen Y. T., McGregor D., Jungbluth A. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998 Jan;22(1):57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Goovaerts G., Buyssens N. Nevus cell maturation or atrophy? Am J Dermatopathol. 1988 Feb;10(1):20–27. doi: 10.1097/00000372-198802000-00003. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Hofbauer G. F., Kamarashev J., Geertsen R., Böni R., Dummer R. Tyrosinase immunoreactivity in formalin-fixed, paraffin-embedded primary and metastatic melanoma: frequency and distribution. J Cutan Pathol. 1998 Apr;25(4):204–209. doi: 10.1111/j.1600-0560.1998.tb01720.x. [DOI] [PubMed] [Google Scholar]
- Jungbluth A. A., Busam K. J., Gerald W. L., Stockert E., Coplan K. A., Iversen K., MacGregor D. P., Old L. J., Chen Y. T. A103: An anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol. 1998 May;22(5):595–602. doi: 10.1097/00000478-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Kanitakis J., Hermier C., Chouvet B., Thivolet J. Reactivity of HMB-45 monoclonal antibody with sweat-gland tumours of the skin. Acta Derm Venereol. 1991;71(5):426–428. [PubMed] [Google Scholar]
- Kaufmann O., Koch S., Burghardt J., Audring H., Dietel M. Tyrosinase, melan-A, and KBA62 as markers for the immunohistochemical identification of metastatic amelanotic melanomas on paraffin sections. Mod Pathol. 1998 Aug;11(8):740–746. [PubMed] [Google Scholar]
- Kunter U., Buer J., Probst M., Duensing S., Dallmann I., Grosse J., Kirchner H., Schluepen E. M., Volkenandt M., Ganser A. Peripheral blood tyrosinase messenger RNA detection and survival in malignant melanoma. J Natl Cancer Inst. 1996 May 1;88(9):590–594. doi: 10.1093/jnci/88.9.590. [DOI] [PubMed] [Google Scholar]
- Longacre T. A., Egbert B. M., Rouse R. V. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol. 1996 Dec;20(12):1489–1500. doi: 10.1097/00000478-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Bröcker E. B. Immunohistochemistry in the evaluation of melanocytic tumors. Semin Diagn Pathol. 1993 Feb;10(1):76–91. [PubMed] [Google Scholar]
- Shivers S. C., Wang X., Li W., Joseph E., Messina J., Glass L. F., DeConti R., Cruse C. W., Berman C., Fenske N. A. Molecular staging of malignant melanoma: correlation with clinical outcome. JAMA. 1998 Oct 28;280(16):1410–1415. doi: 10.1001/jama.280.16.1410. [DOI] [PubMed] [Google Scholar]
- Skelton H. G., 3rd, Smith K. J., Barrett T. L., Lupton G. P., Graham J. H. HMB-45 staining in benign and malignant melanocytic lesions. A reflection of cellular activation. Am J Dermatopathol. 1991 Dec;13(6):543–550. doi: 10.1097/00000372-199113060-00004. [DOI] [PubMed] [Google Scholar]
- Smoller B. R., McNutt N. S., Hsu A. HMB-45 recognizes stimulated melanocytes. J Cutan Pathol. 1989 Apr;16(2):49–53. doi: 10.1111/j.1600-0560.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Stevens G. L., Scheer W. D., Levine E. A. Detection of tyrosinase mRNA from the blood of melanoma patients. Cancer Epidemiol Biomarkers Prev. 1996 Apr;5(4):293–296. [PubMed] [Google Scholar]
- Takahashi K., Isobe T., Ohtsuki Y., Akagi T., Sonobe H., Okuyama T. Immunohistochemical study on the distribution of alpha and beta subunits of S-100 protein in human neoplasm and normal tissues. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):385–396. doi: 10.1007/BF02889881. [DOI] [PubMed] [Google Scholar]
- Vanstapel M. J., Gatter K. C., de Wolf-Peeters C., Mason D. Y., Desmet V. D. New sites of human S-100 immunoreactivity detected with monoclonal antibodies. Am J Clin Pathol. 1986 Feb;85(2):160–168. doi: 10.1093/ajcp/85.2.160. [DOI] [PubMed] [Google Scholar]