Abstract
Aims—The regulation of cell proliferation is a key event in normal development, pathophysiological responses to injury, and tumorigenesis. The orderly progression of cells through the cell cycle depends on a finely tuned balance between the concentrations of activated cyclins and cyclin dependent kinases. This study was undertaken to compare the expression of cell cycle regulators in benign and malignant melanocytic lesions during tumour progression.
Methods—Immunohistochemistry was used to analyse 49 primary cutaneous malignant melanomas, 18 metastatic melanomas, and 12 histologically confirmed naevus cell naevi for their expression of cyclins (A, B1, D1, D2, D3, and E) and cyclin dependent kinases (CDK1, CDK2, and CDK4).
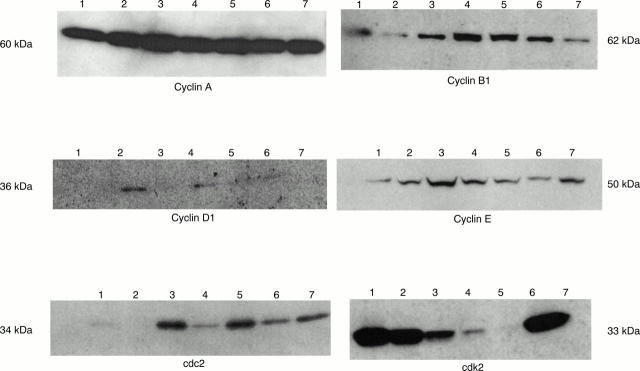
Results—Cyclin E and CDK2 had the highest expression patterns in human cutaneous melanomas and metastases and correlated positively with histological type and tumour stage. Cyclins B1, D2, and D3 had significantly increased expression in metastases, but normal or even decreased expression in primary melanomas. However, cyclins A and D1, and CDK1 and CDK4 were expressed very weakly in situ with no significant differences between naevi, melanomas, or metastases, and there was no correlation with histopathological staging. The specificity of recognition by the antibodies used was confirmed by western blotting on a panel of seven human melanoma cell lines. Cyclins A, B, and E were expressed by all seven, whereas cyclin D1 was detectable in six of seven and CDK2 and cdc2 were present in five of seven lines analysed.
Conclusions—Taken together, this study demonstrated a significant increase of cyclin E and CDK2 expression during tumour progression in malignant melanomas.
Key Words: melanoma • immunohistology • naevus • cyclin E • cyclin dependent kinase 2 • cyclin B1
Full Text
The Full Text of this article is available as a PDF (244.2 KB).
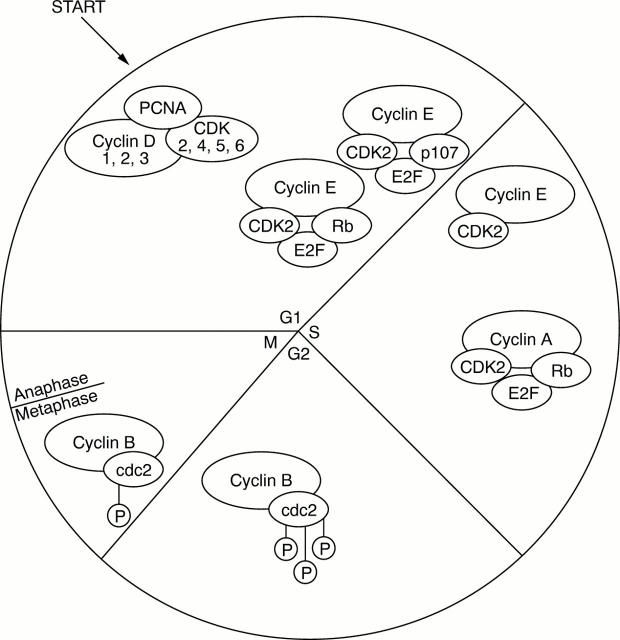
Figure 1 Simplified scheme of the different phases of the cell cycle and their components. Not shown in the scheme are the inhibitors of the cyclin–CDK complexes (p15, p16, p18, p21, and p27) and other cyclin activating kinases and phosphatases.
Figure 2 Western blot analysis using monoclonal antibodies listed in table 2. Seven human melanoma lines were analysed: 1, SK-Mel-23; 2, WM 98–1; 3, UKRV-Mel 4; 5, SK-Mel-37; 6, UKRV-Mel-2; and 7, MV3.
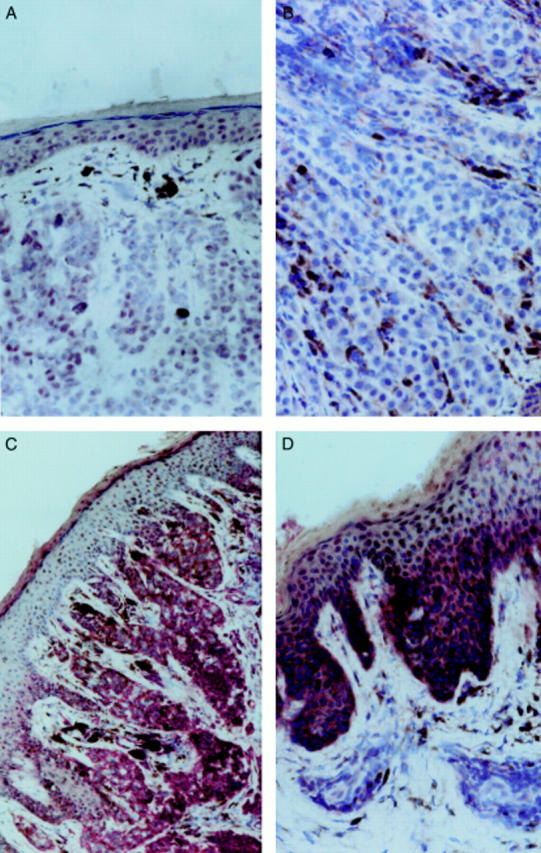
Figure 3 Immunohistological staining of tissue sections using the alkaline phosphatase antialkaline phosphatase (APAAP) technique. (A) A primary melanoma stained with an anticyclin B1 antibody (magnification, x20) and (B) a metastasis stained with anticyclin D3 (magnification, x80). (C) Immunohistological staining of a primary melanoma with an anticyclin E (magnification, x20) and (D) with an anti-CDK2 monoclonal antibody (magnification, x40)
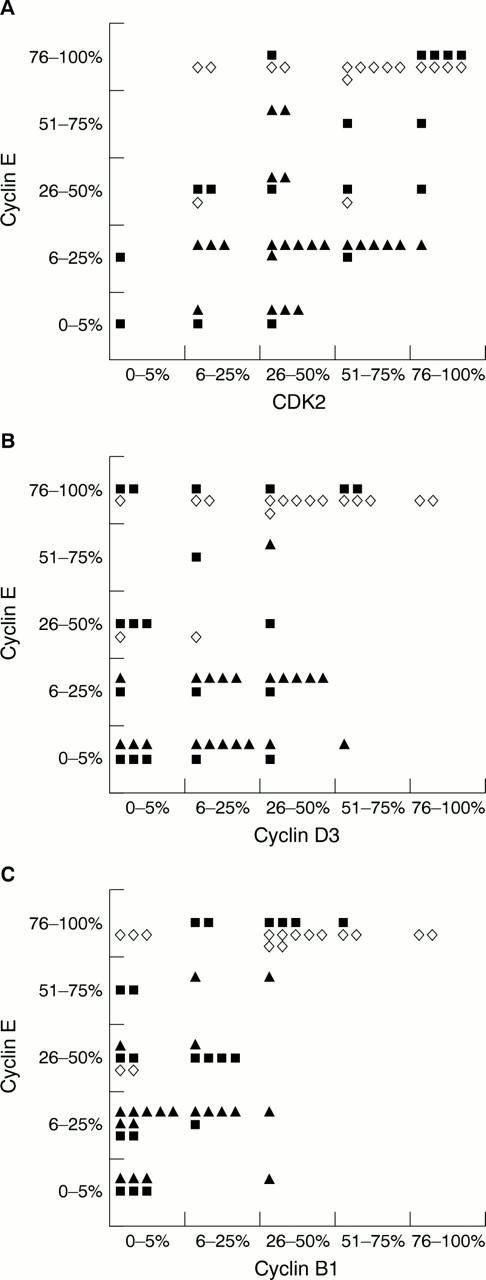
Figure 4 Correlation of cyclin E expression with (A) CDK2, (B) cyclin D3, and (C) cyclin B1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bales E. S., Dietrich C., Bandyopadhyay D., Schwahn D. J., Xu W., Didenko V., Leiss P., Conrad N., Pereira-Smith O., Orengo I. High levels of expression of p27KIP1 and cyclin E in invasive primary malignant melanomas. J Invest Dermatol. 1999 Dec;113(6):1039–1046. doi: 10.1046/j.1523-1747.1999.00812.x. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Lukas J., Strauss M., Bartek J. Cyclin D1 oncoprotein aberrantly accumulates in malignancies of diverse histogenesis. Oncogene. 1995 Feb 16;10(4):775–778. [PubMed] [Google Scholar]
- Bosl G. J., Ilson D. H., Rodriguez E., Motzer R. J., Reuter V. E., Chaganti R. S. Clinical relevance of the i(12p) marker chromosome in germ cell tumors. J Natl Cancer Inst. 1994 Mar 2;86(5):349–355. doi: 10.1093/jnci/86.5.349. [DOI] [PubMed] [Google Scholar]
- Bringuier P. P., Tamimi Y., Schuuring E., Schalken J. Expression of cyclin D1 and EMS1 in bladder tumours; relationship with chromosome 11q13 amplification. Oncogene. 1996 Apr 18;12(8):1747–1753. [PubMed] [Google Scholar]
- Buckley M. F., Sweeney K. J., Hamilton J. A., Sini R. L., Manning D. L., Nicholson R. I., deFazio A., Watts C. K., Musgrove E. A., Sutherland R. L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993 Aug;8(8):2127–2133. [PubMed] [Google Scholar]
- Bártek J., Stasková Z., Draetta G., Lukás J. Molecular pathology of the cell cycle in human cancer cells. Stem Cells. 1993 May;11 (Suppl 1):51–58. doi: 10.1002/stem.5530110611. [DOI] [PubMed] [Google Scholar]
- Callender T., el-Naggar A. K., Lee M. S., Frankenthaler R., Luna M. A., Batsakis J. G. PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer. 1994 Jul 1;74(1):152–158. doi: 10.1002/1097-0142(19940701)74:1<152::aid-cncr2820740124>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Courjal F., Louason G., Speiser P., Katsaros D., Zeillinger R., Theillet C. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. Int J Cancer. 1996 Aug 22;69(4):247–253. doi: 10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Dutta A., Chandra R., Leiter L. M., Lester S. Cyclins as markers of tumor proliferation: immunocytochemical studies in breast cancer. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5386–5390. doi: 10.1073/pnas.92.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Weinberg R. A. Tumor suppressor genes. Curr Opin Genet Dev. 1994 Feb;4(1):135–141. doi: 10.1016/0959-437x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Inohara S., Kitagawa K., Kitano Y. Expression of cyclin D1 and p53 protein in various malignant skin tumors. Dermatology. 1996;192(2):94–98. doi: 10.1159/000246329. [DOI] [PubMed] [Google Scholar]
- Jiang W., Kahn S. M., Tomita N., Zhang Y. J., Lu S. H., Weinstein I. B. Amplification and expression of the human cyclin D gene in esophageal cancer. Cancer Res. 1992 May 15;52(10):2980–2983. [PubMed] [Google Scholar]
- Kamb A. Cell-cycle regulators and cancer. Trends Genet. 1995 Apr;11(4):136–140. doi: 10.1016/s0168-9525(00)89027-7. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K., O'Leary N., Molnar G., Lees E., Fingert H. J., Pardee A. B. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 1994 Jan 15;54(2):380–385. [PubMed] [Google Scholar]
- Keyomarsi K., Pardee A. B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammie G. A., Fantl V., Smith R., Schuuring E., Brookes S., Michalides R., Dickson C., Arnold A., Peters G. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene. 1991 Mar;6(3):439–444. [PubMed] [Google Scholar]
- Leach F. S., Elledge S. J., Sherr C. J., Willson J. K., Markowitz S., Kinzler K. W., Vogelstein B. Amplification of cyclin genes in colorectal carcinomas. Cancer Res. 1993 May 1;53(9):1986–1989. [PubMed] [Google Scholar]
- Li S. F., Shiozawa T., Nakayama K., Nikaido T., Fujii S. Stepwise abnormality of sex steroid hormone receptors, tumor suppressor gene products (p53 and Rb), and cyclin E in uterine endometrioid carcinoma. Cancer. 1996 Jan 15;77(2):321–329. doi: 10.1002/(SICI)1097-0142(19960115)77:2<321::AID-CNCR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Maelandsmo G. M., Flørenes V. A., Hovig E., Oyjord T., Engebraaten O., Holm R., Børresen A. L., Fodstad O. Involvement of the pRb/p16/cdk4/cyclin D1 pathway in the tumorigenesis of sporadic malignant melanomas. Br J Cancer. 1996 Apr;73(8):909–916. doi: 10.1038/bjc.1996.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Arnold A. Cyclins and oncogenesis. Biochim Biophys Acta. 1993 May 25;1155(1):63–78. doi: 10.1016/0304-419x(93)90022-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Zukerberg L., Togawa K., Meltzer S. J., Nishihara T., Rustgi A. K. Human cyclin D1 oncogene and esophageal squamous cell carcinoma. Cancer. 1995 Aug 15;76(4):541–549. doi: 10.1002/1097-0142(19950815)76:4<541::aid-cncr2820760402>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Li S. F., Shiozawa T., Fujii S. Coabnormal expression of cyclin D1 and p53 protein in human uterine endometrial carcinomas. Cancer. 1996 Sep 15;78(6):1248–1253. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1248::AID-CNCR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Paterlini P., Flejou J. F., De Mitri M. S., Pisi E., Franco D., Bréchot C. Structure and expression of the cyclin A gene in human primary liver cancer. Correlation with flow cytometric parameters. J Hepatol. 1995 Jul;23(1):47–52. doi: 10.1016/0168-8278(95)80310-6. [DOI] [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Resnitzky D., Gossen M., Bujard H., Reed S. I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994 Mar;14(3):1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Makki A., Stahr C., van Dyck A., Wanner R., Scheffer G. L., Flens M. J., Scheper R., Henz B. M. Membrane transport proteins associated with drug resistance expressed in human melanoma. Am J Pathol. 1995 Dec;147(6):1545–1552. [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Tang L., Li G., Tron V. A., Trotter M. J., Ho V. C. Expression of cell cycle regulators in human cutaneous malignant melanoma. Melanoma Res. 1999 Apr;9(2):148–154. doi: 10.1097/00008390-199904000-00006. [DOI] [PubMed] [Google Scholar]
- Volm M., Koomägi R., Stammler G., Rittgen W., Zintl F., Sauerbrey A. Prognostic implications of cyclins (D1, E, A), cyclin-dependent kinases (CDK2, CDK4) and tumor-suppressor genes (pRB, p16INK4A) in childhood acute lymphoblastic leukemia. Int J Cancer. 1997 Oct 21;74(5):508–512. doi: 10.1002/(sici)1097-0215(19971021)74:5<508::aid-ijc5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wang A., Yoshimi N., Suzui M., Yamauchi A., Tarao M., Mori H. Different expression patterns of cyclins A, D1 and E in human colorectal cancer. J Cancer Res Clin Oncol. 1996;122(2):122–126. doi: 10.1007/BF01226270. [DOI] [PubMed] [Google Scholar]
- Wang Y., Becker D. Differential expression of the cyclin-dependent kinase inhibitors p16 and p21 in the human melanocytic system. Oncogene. 1996 Mar 7;12(5):1069–1075. [PubMed] [Google Scholar]
- Weinstat-Saslow D., Merino M. J., Manrow R. E., Lawrence J. A., Bluth R. F., Wittenbel K. D., Simpson J. F., Page D. L., Steeg P. S. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med. 1995 Dec;1(12):1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Jiang W., Chen C. J., Lee C. S., Kahn S. M., Santella R. M., Weinstein I. B. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1993 Oct 29;196(2):1010–1016. doi: 10.1006/bbrc.1993.2350. [DOI] [PubMed] [Google Scholar]