Abstract
Background/Aims—Killer inhibitory receptors (KIR) have a modulating effect on the cytotoxic functions of natural killer (NK) cells and T cells. Because lymphoma cells often have the same receptors as their non-neoplastic counterparts, this study investigated the expression of KIR on well defined groups of NK and T cell lymphomas, with and without a cytotoxic phenotype, from different sites of origin.
Methods—Nine CD56+/CD3- NK cell lymphomas, 29 CD3+/CD56- T cell lymphomas with a cytotoxic phenotype, and 19 T cell lymphomas without a cytotoxic phenotype were stained for KIR using monoclonal antibodies specific for CD94, CD158a, and CD158b. In addition, the expression of KIR was studied on normal lymphoid tissues.
Results—KIR expression was seen in five of nine true NK cell lymphomas including three of four nasal, one of four cutaneous, and one of one intestinal lymphoma nasal type. Double staining for CD56 and CD94 in normal lymphoid tissues revealed that KIR was predominantly expressed by CD56+ NK cells and sporadically on CD8+ T cells. Moreover, enteropathy-type T cell lymphomas with a cytotoxic phenotype showed KIR expression (three cases expressing CD94 and one case expressing CD158a). All nodal and extranodal non-intestinal T cell lymphomas with or without a cytotoxic phenotype lacked expression of KIR.
Conclusions—These results show that KIR expression is restricted to CD56+/CD3- true NK cell lymphomas originating from the nose, gut, and skin, as well as in a subset of extranodal T cell lymphomas originating from the small intestine, which possessed a cytotoxic phenotype. Thus, the presence of KIR on NK/T cell lymphomas seems to mimic the distribution of KIR found on NK and T cells in normal lymphoid tissue.
Key Words: lymphoma • granzyme B • killer inhibitory receptors • CD94
Full Text
The Full Text of this article is available as a PDF (183.3 KB).
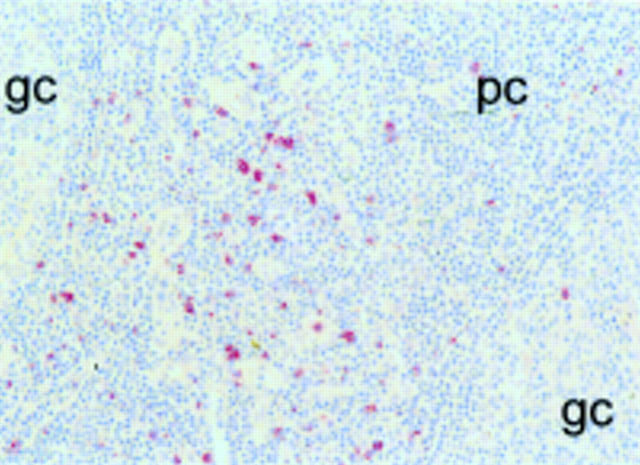
Figure 1 Staining of a tonsil for CD94. CD94 positive cells are predominantly detected in the paracortical areas of tonsils. gc, germinal centre; pc, paracortical area.
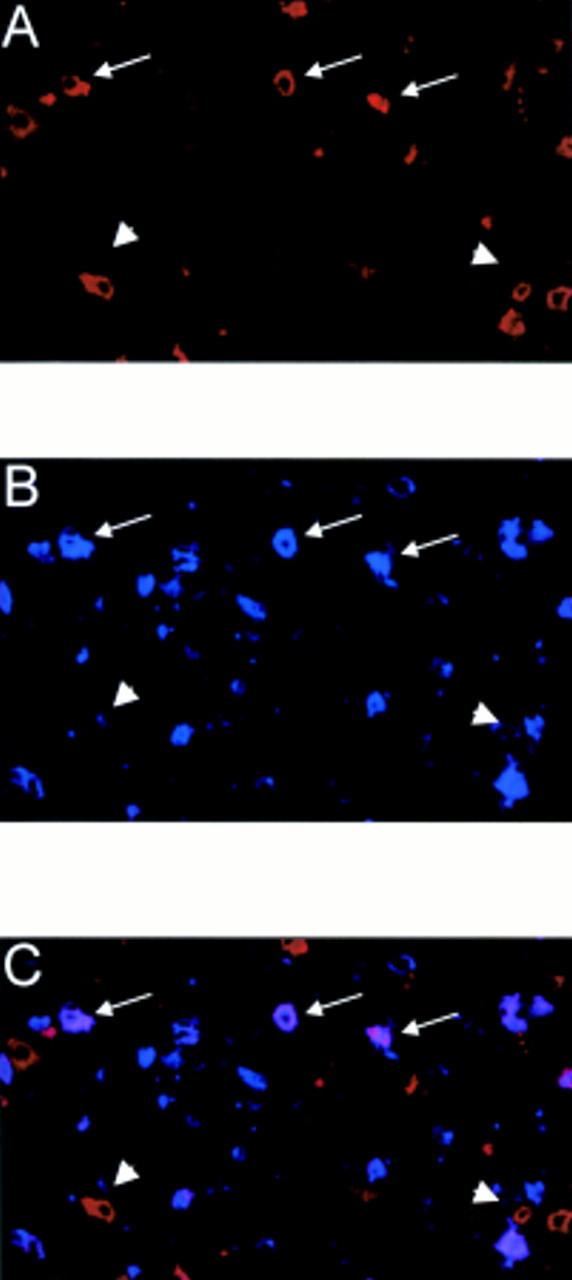
Figure 2 Immunofluorescent double staining for (A) CD56 (red) and (B) CD94 (blue) on normal tonsil. (C) CD56+/CD94+ natural killer (NK) cells (purple) are indicated by arrows, whereas arrowheads indicate CD56+/CD94- NK cells.
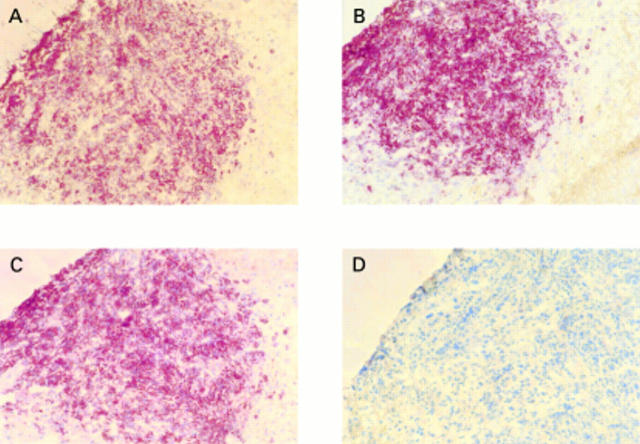
Figure 3 (A) Killer cell inhibitory receptor (KIR) expression on a CD56+/CD3- nasal natural killer cell lymphoma. In addition, neoplastic cells were (B) positive for CD94 and (C) CD158a, but (D) negative for CD158b.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Nagler-Anderson C., O'Brien C., Levine H., Watkins S., Slayter H. S., Blue M. L., Schlossman S. F. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol. 1990 Jan 15;144(2):574–582. [PubMed] [Google Scholar]
- Azuma M., Phillips J. H., Lanier L. L. CD28- T lymphocytes. Antigenic and functional properties. J Immunol. 1993 Feb 15;150(4):1147–1159. [PubMed] [Google Scholar]
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989 Dec 20;125(1-2):279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991 Mar 1;137(1):103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- Dukers D. F., ten Berge R. L., Oudejans J. J., Pulford K., Hayes D., Miseré J. F., Ossenkoppele G. J., Jaspars L. H., Willemze R., Meijer C. J. A cytotoxic phenotype does not predict clinical outcome in anaplastic large cell lymphomas. J Clin Pathol. 1999 Feb;52(2):129–136. doi: 10.1136/jcp.52.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss H. D., Anagnostopoulos I., Araujo I., Assaf C., Demel G., Kummer J. A., Hummel M., Stein H. Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood. 1996 Nov 15;88(10):4005–4011. [PubMed] [Google Scholar]
- Haedicke W., Ho F. C., Chott A., Moretta L., Rüdiger T., Ott G., Müller-Hermelink H. K. Expression of CD94/NKG2A and killer immunoglobulin-like receptors in NK cells and a subset of extranodal cytotoxic T-cell lymphomas. Blood. 2000 Jun 1;95(11):3628–3630. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Diebold J., Flandrin G., Muller-Hermelink H. K., Vardiman J., Lister T. A., Bloomfield C. D. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999 Dec;17(12):3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Jiwa N. M., Kanavaros P., De Bruin P. C., van der Valk P., Horstman A., Vos W., Mullink H., Walboomers J. M., Meijer C. J. Presence of Epstein-Barr virus harbouring small and intermediate-sized cells in Hodgkin's disease. Is there a relationship with Reed-Sternberg cells? J Pathol. 1993 Jun;170(2):129–136. doi: 10.1002/path.1711700206. [DOI] [PubMed] [Google Scholar]
- Krenacs L., Wellmann A., Sorbara L., Himmelmann A. W., Bagdi E., Jaffe E. S., Raffeld M. Cytotoxic cell antigen expression in anaplastic large cell lymphomas of T- and null-cell type and Hodgkin's disease: evidence for distinct cellular origin. Blood. 1997 Feb 1;89(3):980–989. [PubMed] [Google Scholar]
- Kummer J. A., Kamp A. M., van Katwijk M., Brakenhoff J. P., Radosević K., van Leeuwen A. M., Borst J., Verweij C. L., Hack C. E. Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods. 1993 Jul 6;163(1):77–83. doi: 10.1016/0022-1759(93)90241-x. [DOI] [PubMed] [Google Scholar]
- Kummer J. A., Vermeer M. H., Dukers D., Meijer C. J., Willemze R. Most primary cutaneous CD30-positive lymphoproliferative disorders have a CD4-positive cytotoxic T-cell phenotype. J Invest Dermatol. 1997 Nov;109(5):636–640. doi: 10.1111/1523-1747.ep12337633. [DOI] [PubMed] [Google Scholar]
- Mingari M. C., Ponte M., Cantoni C., Vitale C., Schiavetti F., Bertone S., Bellomo R., Cappai A. T., Biassoni R. HLA-class I-specific inhibitory receptors in human cytolytic T lymphocytes: molecular characterization, distribution in lymphoid tissues and co-expression by individual T cells. Int Immunol. 1997 Apr;9(4):485–491. doi: 10.1093/intimm/9.4.485. [DOI] [PubMed] [Google Scholar]
- Mingari M. C., Schiavetti F., Ponte M., Vitale C., Maggi E., Romagnani S., Demarest J., Pantaleo G., Fauci A. S., Moretta L. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12433–12438. doi: 10.1073/pnas.93.22.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Pende D., Tripodi G., Tambussi G., Viale O., Orengo A., Barbaresi M., Merli A., Ciccone E. Identification of four subsets of human CD3-CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990 Dec 1;172(6):1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997 Oct;9(5):694–701. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ciccone E., Mingari M. C., Biassoni R., Moretta A. Human natural killer cells: origin, clonality, specificity, and receptors. Adv Immunol. 1994;55:341–380. doi: 10.1016/s0065-2776(08)60513-1. [DOI] [PubMed] [Google Scholar]
- Oudejans J. J., Kummer J. A., Jiwa M., van der Valk P., Ossenkoppele G. J., Kluin P. M., Kluin-Nelemans J. C., Meijer C. J. Granzyme B expression in Reed-Sternberg cells of Hodgkin's disease. Am J Pathol. 1996 Jan;148(1):233–240. [PMC free article] [PubMed] [Google Scholar]
- Oudejans J. J., Kummer J. A., Jiwa M., van der Valk P., Ossenkoppele G. J., Kluin P. M., Kluin-Nelemans J. C., Meijer C. J. Granzyme B expression in Reed-Sternberg cells of Hodgkin's disease. Am J Pathol. 1996 Jan;148(1):233–240. [PMC free article] [PubMed] [Google Scholar]
- Speiser D. E., Valmori D., Rimoldi D., Pittet M. J., Liénard D., Cerundolo V., MacDonald H. R., Cerottini J. C., Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur J Immunol. 1999 Jun;29(6):1990–1999. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Valiante N. M., Uhrberg M., Shilling H. G., Lienert-Weidenbach K., Arnett K. L., D'Andrea A., Phillips J. H., Lanier L. L., Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997 Dec;7(6):739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- Vermeer M. H., Geelen F. A., Kummer J. A., Meijer C. J., Willemze R. Expression of cytotoxic proteins by neoplastic T cells in mycosis fungoides increases with progression from plaque stage to tumor stage disease. Am J Pathol. 1999 Apr;154(4):1203–1210. doi: 10.1016/S0002-9440(10)65372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagtmann N., Biassoni R., Cantoni C., Verdiani S., Malnati M. S., Vitale M., Bottino C., Moretta L., Moretta A., Long E. O. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995 May;2(5):439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Willemze R., Kerl H., Sterry W., Berti E., Cerroni L., Chimenti S., Diaz-Peréz J. L., Geerts M. L., Goos M., Knobler R. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997 Jul 1;90(1):354–371. [PubMed] [Google Scholar]
- Yokoyama W. M. HLA class I specificity for natural killer cell receptor CD94/NKG2A: two for one in more ways than one. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):4791–4794. doi: 10.1073/pnas.95.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin P. C., Connolly C. E., Oudejans J. J., Kummer J. A., Jansen W., McCarthy C. F., Meijer C. J. Enteropathy-associated T-cell lymphomas have a cytotoxic T-cell phenotype. Histopathology. 1997 Oct;31(4):313–317. doi: 10.1046/j.1365-2559.1997.2660862.x. [DOI] [PubMed] [Google Scholar]
- de Bruin P. C., Kummer J. A., van der Valk P., van Heerde P., Kluin P. M., Willemze R., Ossenkoppele G. J., Radaszkiewicz T., Meijer C. J. Granzyme B-expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood. 1994 Dec 1;84(11):3785–3791. [PubMed] [Google Scholar]