Abstract
Advances in molecular biology have increased our knowledge of the biology of preneoplastic lesions in the human lung. The recently published WHO lung tumour classification defines three separate lesions that are regarded as preinvasive neoplasia. These are (1) squamous dysplasia and carcinoma in situ (SD/CIS), (2) atypical adenomatous hyperplasia (AAH), and (3) diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH). SD/CIS is graded in four stages (mild, moderate, severe, and CIS), based upon the distribution of atypical cells and mitotic figures. Most airways showing SD/CIS demonstrate a range of grades; many epithelia are hard to assess and the reproducibility of this complex system remains to be established. Detailed criteria are, however, welcome and provide an objective framework on which to compare various molecular changes. Alterations in gene expression and chromosome structure known to be associated with malignant transformation can be demonstrated in CIS, less so in dysplasias, but also in morphologically normal epithelium. The changes might be sequential, and their frequency and number increase with atypia. Less is known of the "risk of progression" of SD/CIS to invasive "central" bronchial carcinoma. It may take between one and 10 years for invasion to occur, yet the lesion(s) may be reversible if carcinogen exposure ceases.
AAH may be an important precursor lesion for peripheral "parenchymal" adenocarcinoma of the lung: the "adenoma" in an adenoma–carcinoma sequence. There is good morphological evidence that AAH may progress from low to high grade to bronchioloalveolar carcinoma (BAC; a non-invasive lesion by definition). Invasion then develops within BAC and peripheral lung adenocarcinoma evolves. The molecular events associated with this progression are not well understood and studies are hampered by a lack of clear criteria to distinguish high grade AAH from BAC. Nonetheless, as with SD/CIS, the patterns of expression of tumour associated genes are consistent with neoplastic progression. We have little idea of the incidence of AAH in the normal or "smoking" populations. It is found more frequently in cancer bearing lungs, especially in those with adenocarcinoma, and is more common in women. No data are available on the risk of progression of AAH. DIPNECH is an exceptionally rare lesion associated with the development of multiple carcinoid tumours. Almost nothing is known of its biology.
Knowledge of these lesions will be crucial in the design and understanding of lung cancer screening programmes, where it is likely that the morphological and, more importantly perhaps, the molecular characteristics of these lesions will provide useful targets for detection and possibly even treatment.
Key Words: lung cancer • preneoplasia • carcinogenesis
Full Text
The Full Text of this article is available as a PDF (371.8 KB).
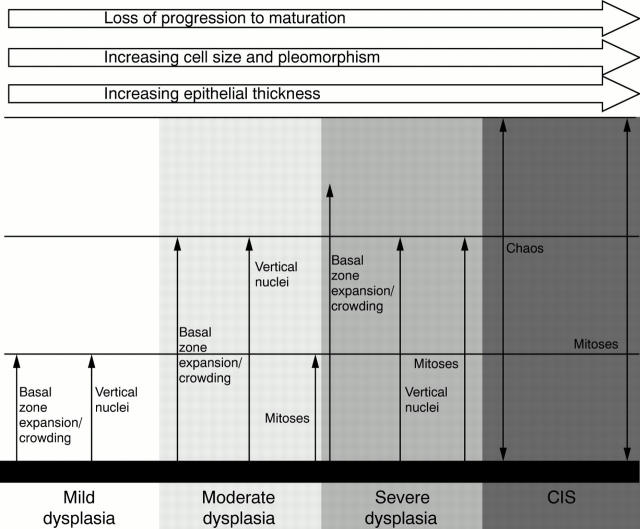
Figure 1 A diagrammatic summary of the WHO criteria13 for bronchial squamous dysplasia/carcinoma in situ (CIS). The distribution of various features is related to the lower, middle, and upper thirds of the multilayered "squamous" epithelium.
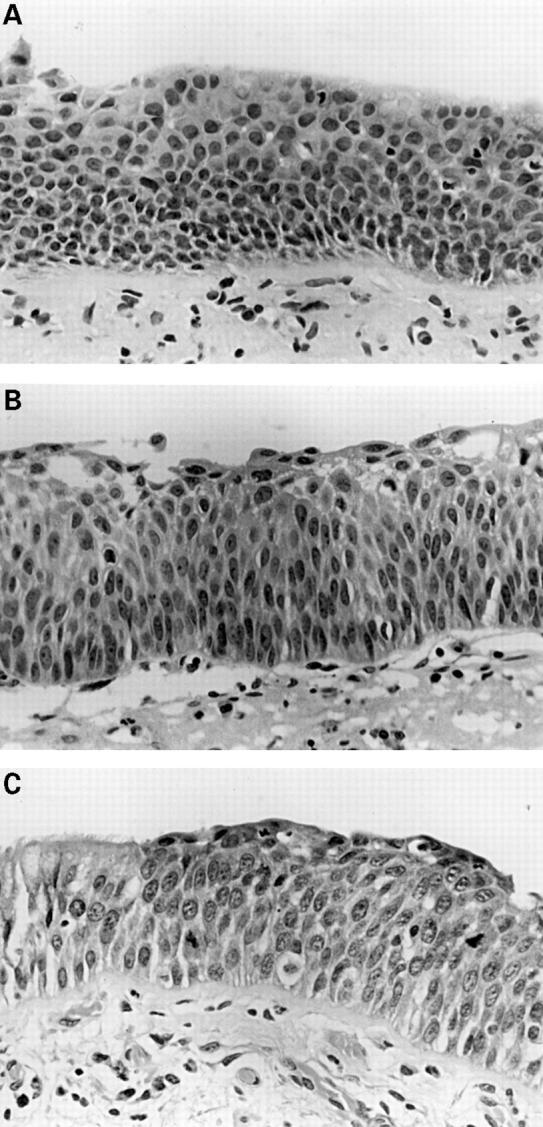
Figure 2 (A) Mild dysplasia: basal zone crowding and minimal cytological atypia. (B) Moderate dysplasia: vertically orientated nuclei occupy the lower two thirds of the epithelium whereas maturation is still clearly visible towards the surface. (C) Severe dysplasia: greater cytological aberration, mitoses are clearly present in the lower two thirds of the epithelium, and there is virtually no maturation. Note the sharp transition from normal respiratory epithelium on the left.
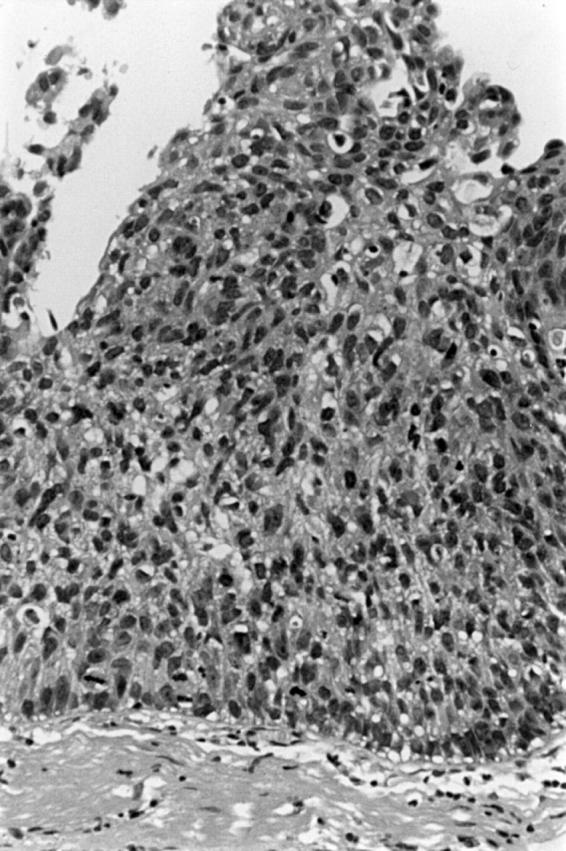
Figure 3 Carcinoma in situ. Full thickness severe cytological atypia with a chaotic appearance.
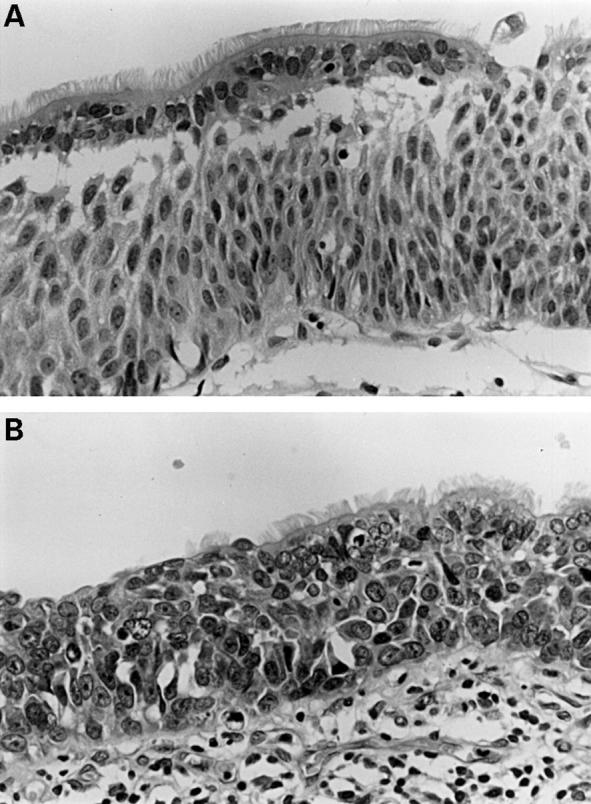
Figure 4 (A) Ciliated respiratory epithelial cells overlying a population showing clear squamous differentiation (intercellular bridges) and prominent vertical orientation of nuclei (moderate dysplasia?) (B) Apart from the persisting superficial ciliated cells, this could be regarded as carcinoma in situ.
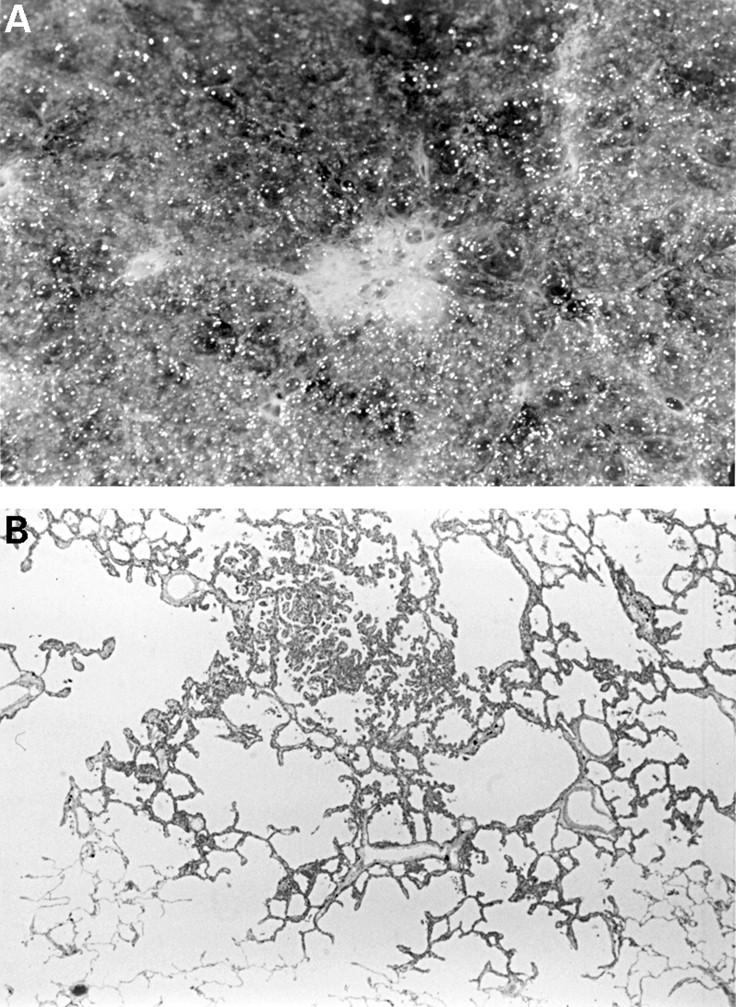
Figure 5 (A) A 4 mm diameter focus of atypical adenomatous hyperplasia (AAH) easily visible on the lung cut surface as a result of abundant collagen in the alveolar walls. (B) AAH lesions are often found in the centriacinar zone, near respiratory bronchioles.
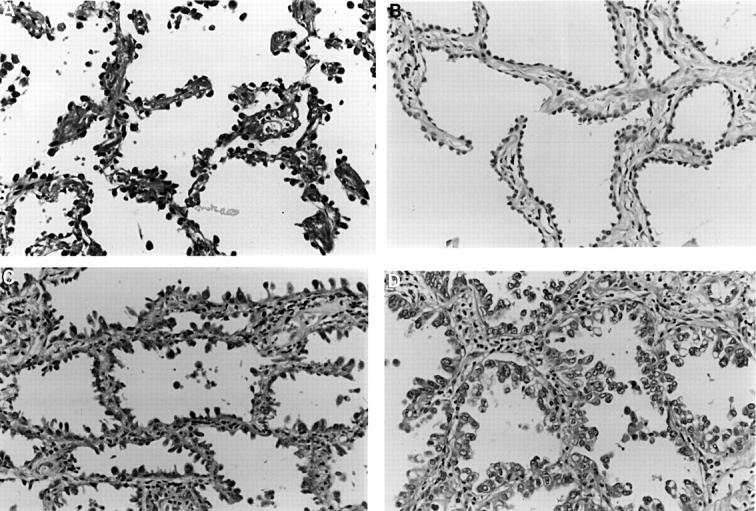
Figure 6 Atypical adenomatous hyperplasia. Low grade lesions show (A) intermittent or (B) complete runs of cuboidal cells lining slightly thickened alveolar walls. More cellular lesions (C) may be larger and have columnar Clara-like cells. Less often, lesions are very cellular, more atypical (D), and very difficult to distinguish from bronchioloalveolar cell carcinoma.
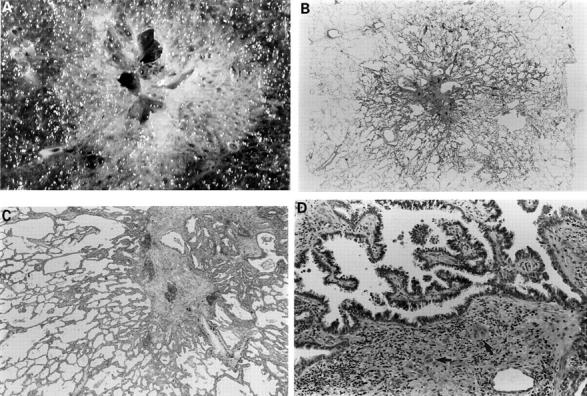
Figure 7 (A) This 1.7 cm lesion with a rather solid and cystic centre has alveolar spaces clearly visible in the periphery. (B) Whole mount of the same lesion. Closer examination (C) shows a periphery typical of atypical adenomatous hyperplasia but the central, solid zone shows (D) papillary bronchioloalveolar-like areas and clear evidence of stromal invasion (arrows).
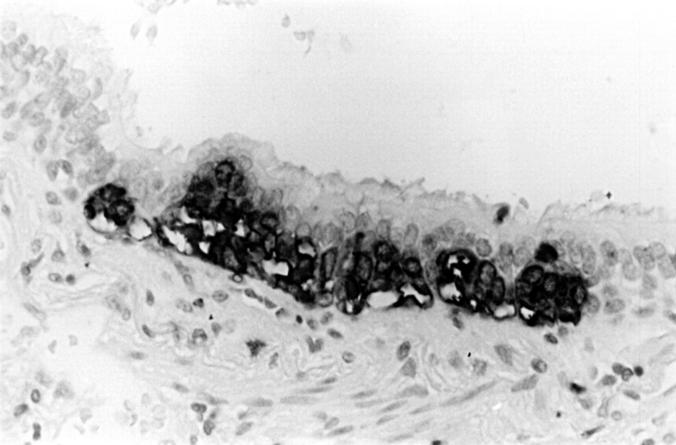
Figure 8 A hyperplastic focus of neuroendocrine cells in the airway epithelium, found in the same lobe as an atypical carcinoid tumour (immunoperoxidase with anti-chromogranin).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH O., STOUT A. P., HAMMOND E. C., GARFINKEL L. Changes in bronchial epithelium in relation to sex, age, residence, smoking and pneumonia. N Engl J Med. 1962 Jul 19;267:111–119. doi: 10.1056/NEJM196207192670301. [DOI] [PubMed] [Google Scholar]
- Aguayo S. M., Miller Y. E., Waldron J. A., Jr, Bogin R. M., Sunday M. E., Staton G. W., Jr, Beam W. R., King T. E., Jr Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med. 1992 Oct 29;327(18):1285–1288. doi: 10.1056/NEJM199210293271806. [DOI] [PubMed] [Google Scholar]
- Anami Y., Matsuno Y., Yamada T., Takeuchi T., Nakayama H., Hirohashi S., Noguchi M. A case of double primary adenocarcinoma of the lung with multiple atypical adenomatous hyperplasia. Pathol Int. 1998 Aug;48(8):634–640. doi: 10.1111/j.1440-1827.1998.tb03962.x. [DOI] [PubMed] [Google Scholar]
- Auerbach O., Hammond E. C., Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking, 1955-1960 vs. 1970-1977. N Engl J Med. 1979 Feb 22;300(8):381–385. doi: 10.1056/NEJM197902223000801. [DOI] [PubMed] [Google Scholar]
- Auerbach O. Pathogenesis of lung cancer. Compr Ther. 1981 Dec;7(12):11–21. [PubMed] [Google Scholar]
- Barsky S. H., Huang S. J., Bhuta S. The extracellular matrix of pulmonary scar carcinomas is suggestive of a desmoplastic origin. Am J Pathol. 1986 Sep;124(3):412–419. [PMC free article] [PubMed] [Google Scholar]
- Bennett W. P., Colby T. V., Travis W. D., Borkowski A., Jones R. T., Lane D. P., Metcalf R. A., Samet J. M., Takeshima Y., Gu J. R. p53 protein accumulates frequently in early bronchial neoplasia. Cancer Res. 1993 Oct 15;53(20):4817–4822. [PubMed] [Google Scholar]
- Brambilla E., Gazzeri S., Lantuejoul S., Coll J. L., Moro D., Negoescu A., Brambilla C. p53 mutant immunophenotype and deregulation of p53 transcription pathway (Bcl2, Bax, and Waf1) in precursor bronchial lesions of lung cancer. Clin Cancer Res. 1998 Jul;4(7):1609–1618. [PubMed] [Google Scholar]
- Brambilla E., Gazzeri S., Moro D., Lantuejoul S., Veyrenc S., Brambilla C. Alterations of Rb pathway (Rb-p16INK4-cyclin D1) in preinvasive bronchial lesions. Clin Cancer Res. 1999 Feb;5(2):243–250. [PubMed] [Google Scholar]
- Brambilla E., Negoescu A., Gazzeri S., Lantuejoul S., Moro D., Brambilla C., Coll J. L. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am J Pathol. 1996 Dec;149(6):1941–1952. [PMC free article] [PubMed] [Google Scholar]
- Béjui-Thivolet F., Liagre N., Chignol M. C., Chardonnet Y., Patricot L. M. Detection of human papillomavirus DNA in squamous bronchial metaplasia and squamous cell carcinomas of the lung by in situ hybridization using biotinylated probes in paraffin-embedded specimens. Hum Pathol. 1990 Jan;21(1):111–116. doi: 10.1016/0046-8177(90)90082-g. [DOI] [PubMed] [Google Scholar]
- Carey F. A., Salter D. M., Kerr K. M., Lamb D. An investigation into the role of human papillomavirus in endobronchial papillary squamous tumours. Respir Med. 1990 Nov;84(6):445–447. doi: 10.1016/s0954-6111(08)80107-2. [DOI] [PubMed] [Google Scholar]
- Carey F. A., Wallace W. A., Fergusson R. J., Kerr K. M., Lamb D. Alveolar atypical hyperplasia in association with primary pulmonary adenocarcinoma: a clinicopathological study of 10 cases. Thorax. 1992 Dec;47(12):1041–1043. doi: 10.1136/thx.47.12.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. D., Kerr K. M. The association between atypical adenomatous hyperplasia and primary lung cancer. Br J Cancer. 2000 Sep;83(5):632–636. doi: 10.1054/bjoc.2000.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. R., Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992 Sep 15;70(6 Suppl):1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Colby T. V., Wistuba I. I., Gazdar A. Precursors to pulmonary neoplasia. Adv Anat Pathol. 1998 Jul;5(4):205–215. doi: 10.1097/00125480-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Cooper C. A., Carby F. A., Bubb V. J., Lamb D., Kerr K. M., Wyllie A. H. The pattern of K-ras mutation in pulmonary adenocarcinoma defines a new pathway of tumour development in the human lung. J Pathol. 1997 Apr;181(4):401–404. doi: 10.1002/(SICI)1096-9896(199704)181:4<401::AID-PATH799>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Endo C., Sagawa M., Sato M., Chen Y., Sakurada A., Aikawa H., Takahashi S., Usuda K., Saito Y., Fujimura S. Sequential loss of heterozygosity in the progression of squamous cell carcinoma of the lung. Br J Cancer. 1998 Sep;78(5):612–615. doi: 10.1038/bjc.1998.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong K. M., Biesterveld E. J., Virmani A., Wistuba I., Sekido Y., Bader S. A., Ahmadian M., Ong S. T., Rassool F. V., Zimmerman P. V. FHIT and FRA3B 3p14.2 allele loss are common in lung cancer and preneoplastic bronchial lesions and are associated with cancer-related FHIT cDNA splicing aberrations. Cancer Res. 1997 Jun 1;57(11):2256–2267. [PubMed] [Google Scholar]
- Fraire A. E., Greenberg S. D. Carcinoma and diffuse interstitial fibrosis of lung. Cancer. 1973 May;31(5):1078–1086. doi: 10.1002/1097-0142(197305)31:5<1078::aid-cncr2820310507>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- George P. J. Fluorescence bronchoscopy for the early detection of lung cancer. Thorax. 1999 Feb;54(2):180–183. doi: 10.1136/thx.54.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Miyamoto H., Ito T., Kameda Y., Nakamura N., Kubota Y., Kitamura H. Analysis of p21Waf1/Cip1 expression in normal, premalignant, and malignant cells during the development of human lung adenocarcinoma. Am J Pathol. 1997 Aug;151(2):461–470. [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Franzén B., Kato H., Ebihara Y., Auer G. Genesis of squamous cell lung carcinoma. Sequential changes of proliferation, DNA ploidy, and p53 expression. Am J Pathol. 1994 Feb;144(2):296–302. [PMC free article] [PubMed] [Google Scholar]
- Hung J., Kishimoto Y., Sugio K., Virmani A., McIntire D. D., Minna J. D., Gazdar A. F. Allele-specific chromosome 3p deletions occur at an early stage in the pathogenesis of lung carcinoma. JAMA. 1995 Feb 15;273(7):558–563. [PubMed] [Google Scholar]
- Katabami M., Dosaka-Akita H., Honma K., Kimura K., Fujino M., Uchida Y., Mikami H., Ohsaki Y., Kawakami Y., Kikuchi K. p53 and Bcl-2 expression in pneumoconiosis-related pre-cancerous lesions and lung cancers: frequent and preferential p53 expression in pneumoconiotic bronchiolar dysplasias. Int J Cancer. 1998 Feb 9;75(4):504–511. doi: 10.1002/(sici)1097-0215(19980209)75:4<504::aid-ijc3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. M., Lamb D., King G., Kerr K. M. Cell proliferation, cell loss and expression of bcl-2 and p53 in human pulmonary neoplasms. Br J Cancer. 1997;75(4):545–547. doi: 10.1038/bjc.1997.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K. M., Carey F. A., King G., Lamb D. Atypical alveolar hyperplasia: relationship with pulmonary adenocarcinoma, p53, and c-erbB-2 expression. J Pathol. 1994 Dec;174(4):249–256. doi: 10.1002/path.1711740404. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Kameda Y., Ito T., Hayashi H. Atypical adenomatous hyperplasia of the lung. Implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol. 1999 May;111(5):610–622. doi: 10.1093/ajcp/111.5.610. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Kameda Y., Ito T., Hayashi H., Nakamura N., Nakatani Y., Inayama Y., Kanisawa M. Cytodifferentiation of atypical adenomatous hyperplasia and bronchioloalveolar lung carcinoma: immunohistochemical and ultrastructural studies. Virchows Arch. 1997 Dec;431(6):415–424. doi: 10.1007/s004280050118. [DOI] [PubMed] [Google Scholar]
- Kitamura H., Kameda Y., Nakamura N., Inayama Y., Nakatani Y., Shibagaki T., Ito T., Hayashi H., Kimura H., Kanisawa M. Atypical adenomatous hyperplasia and bronchoalveolar lung carcinoma. Analysis by morphometry and the expressions of p53 and carcinoembryonic antigen. Am J Surg Pathol. 1996 May;20(5):553–562. doi: 10.1097/00000478-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Kodama T., Biyajima S., Watanabe S., Shimosato Y. Morphometric study of adenocarcinomas and hyperplastic epithelial lesions in the peripheral lung. Am J Clin Pathol. 1986 Feb;85(2):146–151. doi: 10.1093/ajcp/85.2.146. [DOI] [PubMed] [Google Scholar]
- Kodama T., Biyajima S., Watanabe S., Shimosato Y. Morphometric study of adenocarcinomas and hyperplastic epithelial lesions in the peripheral lung. Am J Clin Pathol. 1986 Feb;85(2):146–151. doi: 10.1093/ajcp/85.2.146. [DOI] [PubMed] [Google Scholar]
- Kohno H., Hiroshima K., Toyozaki T., Fujisawa T., Ohwada H. p53 mutation and allelic loss of chromosome 3p, 9p of preneoplastic lesions in patients with nonsmall cell lung carcinoma. Cancer. 1999 Jan 15;85(2):341–347. [PubMed] [Google Scholar]
- Kurasono Y., Ito T., Kameda Y., Nakamura N., Kitamura H. Expression of cyclin D1, retinoblastoma gene protein, and p16 MTS1 protein in atypical adenomatous hyperplasia and adenocarcinoma of the lung. An immunohistochemical analysis. Virchows Arch. 1998 Mar;432(3):207–215. doi: 10.1007/s004280050157. [DOI] [PubMed] [Google Scholar]
- Lam S., MacAulay C., Hung J., LeRiche J., Profio A. E., Palcic B. Detection of dysplasia and carcinoma in situ with a lung imaging fluorescence endoscope device. J Thorac Cardiovasc Surg. 1993 Jun;105(6):1035–1040. [PubMed] [Google Scholar]
- Madri J. A., Carter D. Scar cancers of the lung: origin and significance. Hum Pathol. 1984 Jul;15(7):625–631. doi: 10.1016/s0046-8177(84)80286-5. [DOI] [PubMed] [Google Scholar]
- Marchetti A., Pellegrini S., Bertacca G., Buttitta F., Gaeta P., Carnicelli V., Nardini V., Griseri P., Chella A., Angeletti C. A. FHIT and p53 gene abnormalities in bronchioloalveolar carcinomas. Correlations with clinicopathological data and K-ras mutations. J Pathol. 1998 Mar;184(3):240–246. doi: 10.1002/(SICI)1096-9896(199803)184:3<240::AID-PATH20>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Miller R. R. Bronchioloalveolar cell adenomas. Am J Surg Pathol. 1990 Oct;14(10):904–912. doi: 10.1097/00000478-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Müller N. L. Neuroendocrine cell hyperplasia and obliterative bronchiolitis in patients with peripheral carcinoid tumors. Am J Surg Pathol. 1995 Jun;19(6):653–658. doi: 10.1097/00000478-199506000-00005. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Nelems B., Evans K. G., Müller N. L., Ostrow D. N. Glandular neoplasia of the lung. A proposed analogy to colonic tumors. Cancer. 1988 Mar 1;61(5):1009–1014. doi: 10.1002/1097-0142(19880301)61:5<1009::aid-cncr2820610525>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Mori M., Chiba R., Takahashi T. Atypical adenomatous hyperplasia of the lung and its differentiation from adenocarcinoma. Characterization of atypical cells by morphometry and multivariate cluster analysis. Cancer. 1993 Oct 15;72(8):2331–2340. doi: 10.1002/1097-0142(19931015)72:8<2331::aid-cncr2820720808>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mori M., Tezuka F., Chiba R., Funae Y., Watanabe M., Nukiwa T., Takahashi T. Atypical adenomatous hyperplasia and adenocarcinoma of the human lung: their heterology in form and analogy in immunohistochemical characteristics. Cancer. 1996 Feb 15;77(4):665–674. [PubMed] [Google Scholar]
- Nadav Y., Pastorino U., Nicholson A. G. Multiple synchronous lung cancers and atypical adenomatous hyperplasia in Li-Fraumeni syndrome. Histopathology. 1998 Jul;33(1):52–54. doi: 10.1046/j.1365-2559.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- Nagamoto N., Saito Y., Ohta S., Sato M., Kanma K., Sagawa M., Takahashi S., Usuda K., Nakada T., Hashimoto K. Relationship of lymph node metastasis to primary tumor size and microscopic appearance of roentgenographically occult lung cancer. Am J Surg Pathol. 1989 Dec;13(12):1009–1013. doi: 10.1097/00000478-198912000-00002. [DOI] [PubMed] [Google Scholar]
- Nagamoto N., Saito Y., Sato M., Sagawa M., Kanma K., Takahashi S., Usuda K., Endo C., Fujimura S., Nakada T. Clinicopathological analysis of 19 cases of isolated carcinoma in situ of the bronchus. Am J Surg Pathol. 1993 Dec;17(12):1234–1243. doi: 10.1097/00000478-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Nagamoto N., Saito Y., Suda H., Imai T., Sato M., Ohta S., Kanma K., Sagawa M., Takahashi S., Usuda K. Relationship between length of longitudinal extension and maximal depth of transmural invasion in roentgenographically occult squamous cell carcinoma of the bronchus (nonpolypoid type). Am J Surg Pathol. 1989 Jan;13(1):11–20. doi: 10.1097/00000478-198901000-00002. [DOI] [PubMed] [Google Scholar]
- Nakanishi K. Alveolar epithelial hyperplasia and adenocarcinoma of the lung. Arch Pathol Lab Med. 1990 Apr;114(4):363–368. [PubMed] [Google Scholar]
- Nakanishi K., Hiroi S., Kawai T., Suzuki M., Torikata C. Argyrophilic nucleolar-organizer region counts and DNA status in bronchioloalveolar epithelial hyperplasia and adenocarcinoma of the lung. Hum Pathol. 1998 Mar;29(3):235–239. doi: 10.1016/s0046-8177(98)90041-7. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Noguchi M., Tsuchiya R., Kodama T., Shimosato Y. Clonal growth of atypical adenomatous hyperplasia of the lung: cytofluorometric analysis of nuclear DNA content. Mod Pathol. 1990 May;3(3):314–320. [PubMed] [Google Scholar]
- Niho S., Yokose T., Suzuki K., Kodama T., Nishiwaki Y., Mukai K. Monoclonality of atypical adenomatous hyperplasia of the lung. Am J Pathol. 1999 Jan;154(1):249–254. doi: 10.1016/S0002-9440(10)65271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuorva K., Soini Y., Kamel D., Autio-Harmainen H., Risteli L., Risteli J., Vähäkangas K., Päkkö P. Concurrent p53 expression in bronchial dysplasias and squamous cell lung carcinomas. Am J Pathol. 1993 Mar;142(3):725–732. [PMC free article] [PubMed] [Google Scholar]
- Ohshima S., Shimizu Y., Takahama M. Detection of c-Ki-ras gene mutation in paraffin sections of adenocarcinoma and atypical bronchioloalveolar cell hyperplasia of human lung. Virchows Arch. 1994;424(2):129–134. doi: 10.1007/BF00193491. [DOI] [PubMed] [Google Scholar]
- Ong S. T., Fong K. M., Bader S. A., Minna J. D., Le Beau M. M., McKeithan T. W., Rassool F. V. Precise localization of the FHIT gene to the common fragile site at 3p14.2 (FRA3B) and characterization of homozygous deletions within FRA3B that affect FHIT transcription in tumor cell lines. Genes Chromosomes Cancer. 1997 Sep;20(1):16–23. doi: 10.1002/(sici)1098-2264(199709)20:1<16::aid-gcc3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Pendleton N., Dixon G. R., Burnett H. E., Occleston N. L., Myskow M. W., Green J. A. Expression of proliferating cell nuclear antigen (PCNA) in dysplasia of the bronchial epithelium. J Pathol. 1993 Jun;170(2):169–172. doi: 10.1002/path.1711700212. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Pueblitz S., Hieger L. R. Expression of p53 and CEA in atypical adenomatous hyperplasia of the lung. Am J Surg Pathol. 1997 Jul;21(7):867–868. doi: 10.1097/00000478-199707000-00018. [DOI] [PubMed] [Google Scholar]
- Saccomanno G., Archer V. E., Auerbach O., Saunders R. P., Brennan L. M. Development of carcinoma of the lung as reflected in exfoliated cells. Cancer. 1974 Jan;33(1):256–270. doi: 10.1002/1097-0142(197401)33:1<256::aid-cncr2820330139>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sagawa M., Saito Y., Fujimura S., Linnoila R. I. K-ras point mutation occurs in the early stage of carcinogenesis in lung cancer. Br J Cancer. 1998 Mar;77(5):720–723. doi: 10.1038/bjc.1998.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp L., Brewster D. The epidemiology of lung cancer in Scotland: a review of trends in incidence, survival and mortality and prospects for prevention. Health Bull (Edinb) 1999 Sep;57(5):318–331. [PubMed] [Google Scholar]
- Shimosato Y., Suzuki A., Hashimoto T., Nishiwaki Y., Kodama T., Yoneyama T., Kameya T. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. Am J Surg Pathol. 1980 Aug;4(4):365–373. doi: 10.1097/00000478-198008000-00005. [DOI] [PubMed] [Google Scholar]
- Slebos R. J., Baas I. O., Clement M. J., Offerhaus G. J., Askin F. B., Hruban R. H., Westra W. H. p53 alterations in atypical alveolar hyperplasia of the human lung. Hum Pathol. 1998 Aug;29(8):801–808. doi: 10.1016/s0046-8177(98)90448-8. [DOI] [PubMed] [Google Scholar]
- Sone S., Takashima S., Li F., Yang Z., Honda T., Maruyama Y., Hasegawa M., Yamanda T., Kubo K., Hanamura K. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998 Apr 25;351(9111):1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- Sozzi G., Miozzo M., Donghi R., Pilotti S., Cariani C. T., Pastorino U., Della Porta G., Pierotti M. A. Deletions of 17p and p53 mutations in preneoplastic lesions of the lung. Cancer Res. 1992 Nov 1;52(21):6079–6082. [PubMed] [Google Scholar]
- Sozzi G., Pastorino U., Moiraghi L., Tagliabue E., Pezzella F., Ghirelli C., Tornielli S., Sard L., Huebner K., Pierotti M. A. Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res. 1998 Nov 15;58(22):5032–5037. [PubMed] [Google Scholar]
- Sozzi G., Sard L., De Gregorio L., Marchetti A., Musso K., Buttitta F., Tornielli S., Pellegrini S., Veronese M. L., Manenti G. Association between cigarette smoking and FHIT gene alterations in lung cancer. Cancer Res. 1997 Jun 1;57(11):2121–2123. [PubMed] [Google Scholar]
- Sozzi G., Tornielli S., Tagliabue E., Sard L., Pezzella F., Pastorino U., Minoletti F., Pilotti S., Ratcliffe C., Veronese M. L. Absence of Fhit protein in primary lung tumors and cell lines with FHIT gene abnormalities. Cancer Res. 1997 Dec 1;57(23):5207–5212. [PubMed] [Google Scholar]
- Spencer H., Dail D. H., Arneaud J. Non-invasive bronchial epithelial papillary tumors. Cancer. 1980 Mar 15;45(6):1486–1497. doi: 10.1002/1097-0142(19800315)45:6<1486::aid-cncr2820450632>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sterner D. J., Mori M., Roggli V. L., Fraire A. E. Prevalence of pulmonary atypical alveolar cell hyperplasia in an autopsy population: a study of 100 cases. Mod Pathol. 1997 May;10(5):469–473. [PubMed] [Google Scholar]
- Sugio K., Kishimoto Y., Virmani A. K., Hung J. Y., Gazdar A. F. K-ras mutations are a relatively late event in the pathogenesis of lung carcinomas. Cancer Res. 1994 Nov 15;54(22):5811–5815. [PubMed] [Google Scholar]
- Sundaresan V., Ganly P., Hasleton P., Rudd R., Sinha G., Bleehen N. M., Rabbitts P. p53 and chromosome 3 abnormalities, characteristic of malignant lung tumours, are detectable in preinvasive lesions of the bronchus. Oncogene. 1992 Oct;7(10):1989–1997. [PubMed] [Google Scholar]
- Suzuki K., Nagai K., Yoshida J., Yokose T., Kodama T., Takahashi K., Nishimura M., Kawasaki H., Yokozaki M., Nishiwaki Y. The prognosis of resected lung carcinoma associated with atypical adenomatous hyperplasia: a comparison of the prognosis of well-differentiated adenocarcinoma associated with atypical adenomatous hyperplasia and intrapulmonary metastasis. Cancer. 1997 Apr 15;79(8):1521–1526. doi: 10.1002/(sici)1097-0142(19970415)79:8<1521::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Ogura T., Yokose T., Nagai K., Mukai K., Kodama T., Nishiwaki Y., Esumi H. Loss of heterozygosity in the tuberous sclerosis gene associated regions in adenocarcinoma of the lung accompanied by multiple atypical adenomatous hyperplasia. Int J Cancer. 1998 Aug 21;79(4):384–389. doi: 10.1002/(sici)1097-0215(19980821)79:4<384::aid-ijc13>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Takahashi K., Yoshida J., Nishimura M., Yokose T., Nishiwaki Y., Nagai K. Synchronous double primary lung carcinomas associated with multiple atypical adenomatous hyperplasia. Lung Cancer. 1998 Feb;19(2):131–139. doi: 10.1016/s0169-5002(97)00082-2. [DOI] [PubMed] [Google Scholar]
- Takigawa N., Segawa Y., Nakata M., Saeki H., Mandai K., Kishino D., Shimono M., Ida M., Eguchi K. Clinical investigation of atypical adenomatous hyperplasia of the lung. Lung Cancer. 1999 Aug;25(2):115–121. doi: 10.1016/s0169-5002(99)00055-0. [DOI] [PubMed] [Google Scholar]
- Tao L. C., Chamberlain D. W., Delarue N. C., Pearson F. G., Donat E. E. Cytologic diagnosis of radiographically occult squamous call carcinoma of the lung. Cancer. 1982 Oct 15;50(8):1580–1586. doi: 10.1002/1097-0142(19821015)50:8<1580::aid-cncr2820500819>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Vähäkangas K. H., Samet J. M., Metcalf R. A., Welsh J. A., Bennett W. P., Lane D. P., Harris C. C. Mutations of p53 and ras genes in radon-associated lung cancer from uranium miners. Lancet. 1992 Mar 7;339(8793):576–580. doi: 10.1016/0140-6736(92)90866-2. [DOI] [PubMed] [Google Scholar]
- Walker C., Robertson L., Myskow M., Dixon G. Expression of the BCL-2 protein in normal and dysplastic bronchial epithelium and in lung carcinomas. Br J Cancer. 1995 Jul;72(1):164–169. doi: 10.1038/bjc.1995.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill H., McDonald J. C. Exposure to crystalline silica and risk of lung cancer: the epidemiological evidence. Thorax. 1996 Jan;51(1):97–102. doi: 10.1136/thx.51.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S. Y., Tsuchiya E., Kasuga T., Sugano H. Incidence of atypical bronchioloalveolar cell hyperplasia of the lung: relation to histological subtypes of lung cancer. Virchows Arch A Pathol Anat Histopathol. 1992;420(6):463–471. doi: 10.1007/BF01600250. [DOI] [PubMed] [Google Scholar]
- Westra W. H., Baas I. O., Hruban R. H., Askin F. B., Wilson K., Offerhaus G. J., Slebos R. J. K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res. 1996 May 1;56(9):2224–2228. [PubMed] [Google Scholar]
- Wistuba I. I., Behrens C., Milchgrub S., Bryant D., Hung J., Minna J. D., Gazdar A. F. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999 Jan 21;18(3):643–650. doi: 10.1038/sj.onc.1202349. [DOI] [PubMed] [Google Scholar]
- Yashima K., Litzky L. A., Kaiser L., Rogers T., Lam S., Wistuba I. I., Milchgrub S., Srivastava S., Piatyszek M. A., Shay J. W. Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res. 1997 Jun 15;57(12):2373–2377. [PubMed] [Google Scholar]