Abstract
Background/Aims—Interleukin 10 (IL-10) is a counterinflammatory peptide implicated in the downregulation of human intestinal immune responses. Enhanced secretion of IL-10 has been documented in gastric biopsy organ culture in Helicobacter pylori infection. This study aimed to define the cellular origins of IL-10 in H pylori associated gastritis, and to determine the effects of endogenous IL-10 on proinflammatory cytokine secretion in vitro.
Methods—Endoscopic biopsies were obtained from the gastric antrum at endoscopy from patients with dyspepsia. Two pairs of antral biopsies were cultured in vitro for 24 hours, one pair in the presence of neutralising anti-IL-10 monoclonal antibody, the other pair as controls. The cytokine content of culture supernatants (tumour necrosis factor α (TNF-α), IL-6, and IL-8) was determined by enzyme linked immunosorbent assay and corrected for biopsy weight. Helicobacter pylori status was established by histology and biopsy urease test, and histopathology graded by the Sydney system. In a subgroup of patients, western blotting was used to establish CagA serological status. Immunohistochemistry for IL-10 was performed on formalin fixed tissues using a combination of microwave antigen retrieval and the indirect avidin–biotin technique. Immunoreactivity was scored semiquantitatively.
Results—In vitro culture was performed in 41 patients: 31 with H pylori positive chronic gastritis and 10 H pylori negative. In vitro secretion of TNF-α, IL-6, and IL-8 for "control" biopsies was significantly higher in H pylori positive versus negative samples, with values of TNF-α and IL-6 correlating with the degree of active and chronic inflammation and being higher in CagA seropositive cases. No evidence for enhanced cytokine secretion was seen in biopsies cocultured in the presence of anti-IL-10 monoclonal antibody. Immunohistochemistry was performed in 29 patients, of whom 13 were H pylori positive. IL-10 immunoreactivity was observed in the surface epithelium in all H pylori positive cases and in 13 of 16 negative cases, especially in areas of surface epithelial degeneration. Lamina propria mononuclear cells (LPMNCs) were positively stained in all H pylori positive cases and in 12 of 16 negative cases, with a significantly greater proportion of positive LPMNCs in the positive group.
Conclusions—This study localised IL-10 protein to the gastric epithelium and LPMNCs. In vitro proinflammatory cytokine secretion was increased in H pylori infection (especially CagA positive infection), but blocking endogenous IL-10 secretion did not significantly increase cytokine secretion. IL-10 is implicated in H pylori infection and might "damp down" local inflammation. The role of gastric IL-10 secretion in determining the clinicopathological outcome of infection merits further study.
Key Words: Helicobacter pylori infection • interleukin 10 • gastritis • immunohistochemistry
Full Text
The Full Text of this article is available as a PDF (263.5 KB).
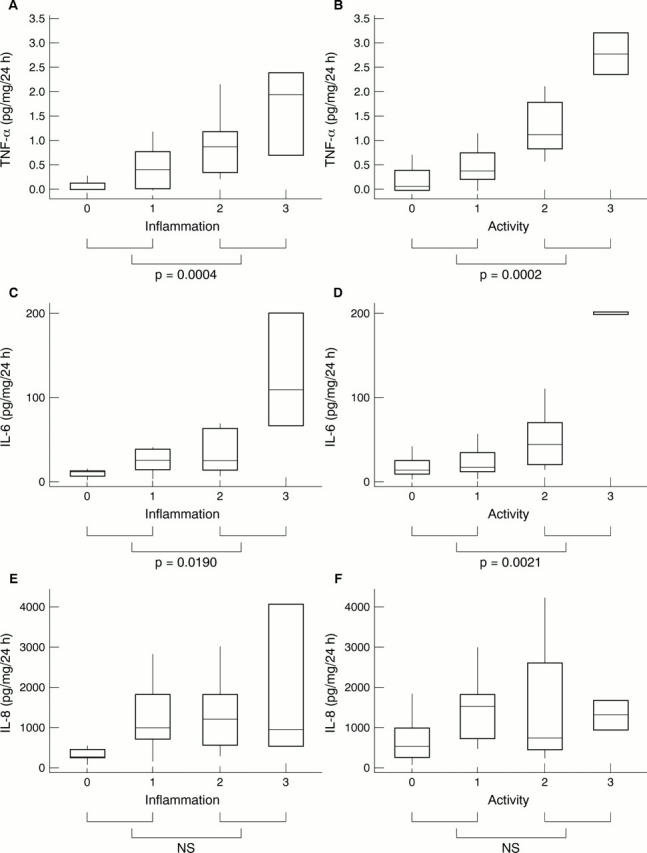
Figure 1 Concentration of tumour necrosis factor α (TNF-α) in 24 hour culture supernatants of "control" biopsies versus (A) antral inflammation (Sydney) score and (B) antral activity (Sydney) score. Concentration of interleukin 6 (IL-6) in 24 hour culture supernatants of "control" biopsies versus (C) antral inflammation (Sydney) score and (D) antral activity (Sydney) score. Concentration of interleukin 8 (IL-8) in 24 hour culture supernatants of "control" biopsies versus (E) antral inflammation (Sydney) score and (F) antral activity (Sydney) score.
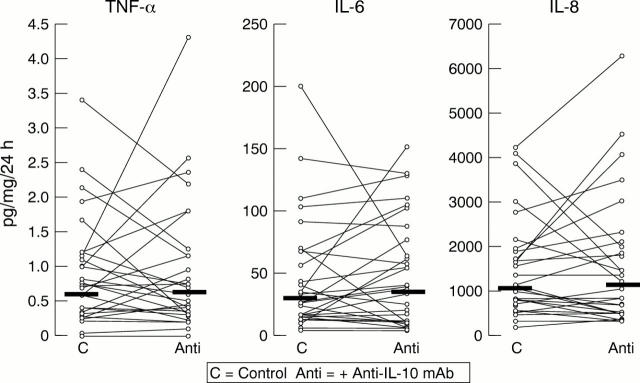
Figure 2 In vitro cytokine secretion for Helicobacter pylori positive patients: control biopsies versus biopsies cocultured in the presence of anti-IL-10 monoclonal antibody (mAb).
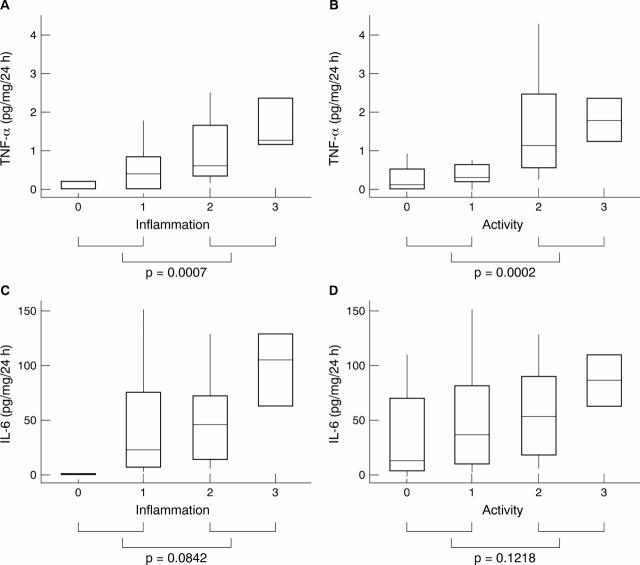
Figure 3 Concentration of tumour necrosis factor α (TNF-α) in 24 hour culture supernatants of biopsies cocultured in the presence of anti-IL-10 monoclonal antibody versus (A) antral inflammation (Sydney) score and (B) antral activity (Sydney) score. Concentration of interleukin 6 (IL-6) in 24 hour culture supernatants of biopsies cocultured in the presence of anti-IL-10 monoclonal antibody versus (C) antral inflammation (Sydney) score and (D) antral activity (Sydney) score.
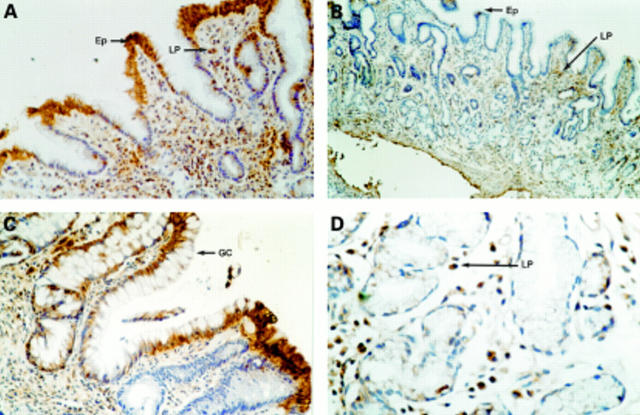
Figure 4 Immunohistochemical localisation of the interleukin 10 (IL-10) protein in formalin fixed gastric mucosal biopsies. (A) Helicobacter pylori positive patient: strong positivity in the surface epithelium (Ep) and frequent lamina propria mononuclear cells (LP). Original magnification, x200. (B) An H pylori negative patient: weak focal positivity in surface epithelium (Ep) and scattered positive lamina propria (LP). Original magnification, x100. (C) A patient with H pylori associated gastritis: strong cytoplasmic positivity of goblet cells (GC) in an area of intestinal metaplasia. Original magnification, x200. (D) A patient with H pylori associated gastritis: high power view of lamina propria showing frequent positive lamina propria mononuclear cells (LP). Original magnification, x400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton J. C. H. pylori virulence factors. Br Med Bull. 1998;54(1):105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- Autschbach F., Braunstein J., Helmke B., Zuna I., Schürmann G., Niemir Z. I., Wallich R., Otto H. F., Meuer S. C. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998 Jul;153(1):121–130. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. J., Lynch N. A., Lynch R. G., Lauricella D. M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(-/-) mice. Am J Pathol. 1998 May;152(5):1377–1386. [PMC free article] [PubMed] [Google Scholar]
- Bodger K., Crabtree J. E. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54(1):139–150. doi: 10.1093/oxfordjournals.bmb.a011664. [DOI] [PubMed] [Google Scholar]
- Bodger K., Wyatt J. I., Heatley R. V. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997 Jun;40(6):739–744. doi: 10.1136/gut.40.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein J., Qiao L., Autschbach F., Schürmann G., Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997 Aug;41(2):215–220. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Wyatt J. I., Trejdosiewicz L. K., Peichl P., Nichols P. H., Ramsay N., Primrose J. N., Lindley I. J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994 Jan;47(1):61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Wyatt J. I., Trejdosiewicz L. K., Peichl P., Nichols P. H., Ramsay N., Primrose J. N., Lindley I. J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994 Jan;47(1):61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elios M. M., Manghetti M., De Carli M., Costa F., Baldari C. T., Burroni D., Telford J. L., Romagnani S., Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997 Jan 15;158(2):962–967. [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Niessner M., Volk B. A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995 Sep;101(3):428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- O'Farrelly C. Just how inflamed is the normal gut? Gut. 1998 May;42(5):603–604. doi: 10.1136/gut.42.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R. M., Jr, Miller G. G., Tham K. T., Perez-Perez G. I., Zhao X., Atherton J. C., Blaser M. J. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995 Dec;73(6):760–770. [PubMed] [Google Scholar]
- Reimund J. M., Dumont S., Muller C. D., Kenney J. S., Kedinger M., Baumann R., Poindron P., Duclos B. In vitro effects of oxpentifylline on inflammatory cytokine release in patients with inflammatory bowel disease. Gut. 1997 Apr;40(4):475–480. doi: 10.1136/gut.40.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Wardle T. D., Hall L., Turnberg L. A. Use of coculture of colonic mucosal biopsies to investigate the release of eicosanoids by inflamed and uninflamed mucosa from patients with inflammatory bowel disease. Gut. 1992 Dec;33(12):1644–1651. doi: 10.1136/gut.33.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996 Jun;110(6):1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Kashima K., Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand J Gastroenterol. 1995 Dec;30(12):1153–1159. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]