Abstract
Aims—The nuclear enzyme DNA topoisomerase II has been shown to be required for chromatin condensation and chromosomal segregation during mitosis; its isoform topo IIα is linked with active cell proliferation in mammalian cells. The aim of this study was to examine the relation of the expression of topo IIα to the biological behaviour of conventional urinary bladder cancer.
Methods—Formalin fixed, paraffin wax embedded tissue from 94 specimens of bladder urothelial cancer were immunohistochemically stained for topo IIα. For each case, a topo IIα index was determined. A similar index had been determined for Ki-67, a known cell proliferation marker. Each case had also been graded, staged, and evaluated for DNA ploidy as well as for p53 and bcl-2 immunoreactivity.
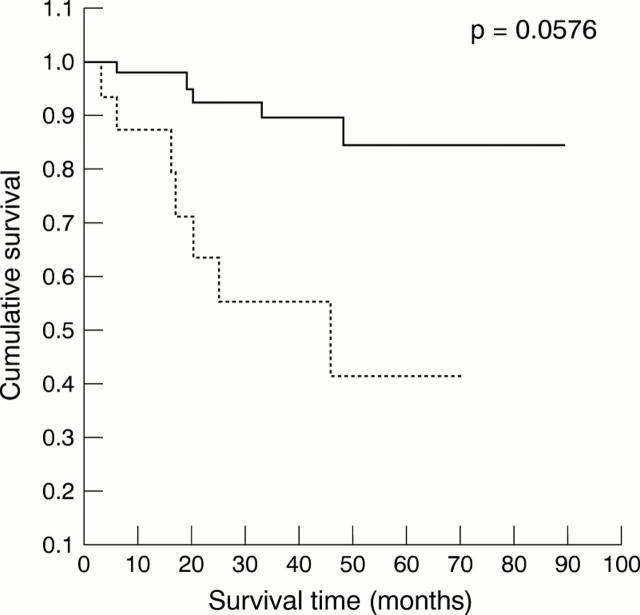
Results—Raised topo IIα expression (in ≥ 10% of malignant nuclei) correlated with two adverse prognosticators—high grade (p = 0.027) and invasion of the muscularis propria (p = 0.013), but with no other evaluated parameter. By multivariate survival analysis using Cox's proportional hazard model, high expression of topo IIα was found to be predictive for worse survival (p = 0.0047). Patients' age, tumour stage, and grade were also retained as independent prognostic factors (p = 0.0349, p = 0.00005, and p = 0.0130, respectively). The negative influence of increased topo IIα immunopositivity on patients' survival was also seen in the subgroup of patients with non-muscle invasive carcinomas (p = 0.0004), in patients with a bcl-2 negative phenotype (p = 0.0330), and in those with low Ki-67 indices (p = 0.0341).
Conclusions—Because topo IIα and Ki-67 failed to demonstrate a significant inter-relation, they appear to be different molecules that both function at separate phases in the complex process of cellular proliferation. The assessment of increased topo IIα immunoreactivity in specimens from urothelial carcinomas might help to select patients (particularly among those with superficial tumours) in the worse prognostic categories for new therapeutic strategies.
Key Words: transitional cell carcinoma • prognosis • DNA topoisomerase IIα
Full Text
The Full Text of this article is available as a PDF (170.6 KB).
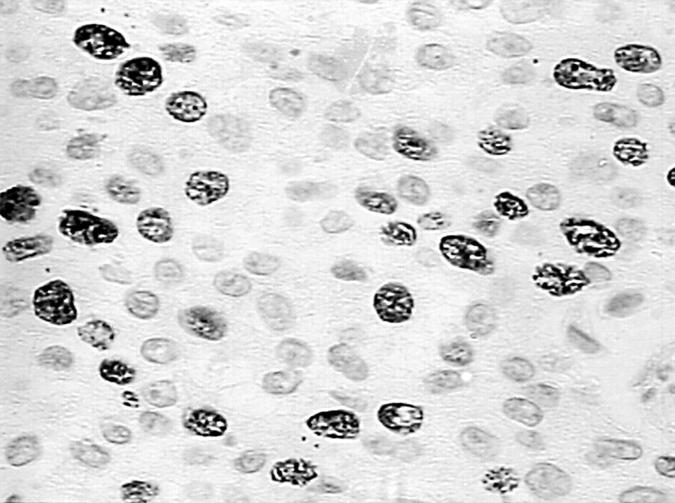
Figure 1 Distinct nuclear positivity for topoisomerase IIα in cancerous cells of a low grade transitional cell carcinoma (avidin–biotin–peroxidase complex; magnification, x400).
Figure 2 Survival of patients with topoisomerase IIα indices ≥ 10% (dotted line) and < 10% (solid line) in human bladder carcinomas.
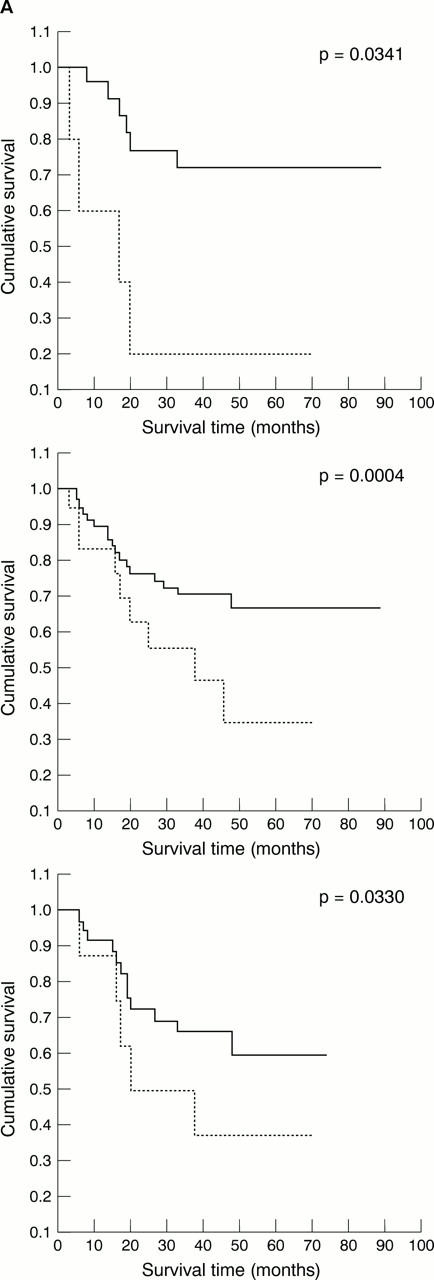
Figure 3 The influence of topoisomerase IIα (topo IIα) expression on the survival of specific patient subgroups. (A) Transitional cell carcinomas (TCCs) with low Ki-67 indices; (B) superficial TCCs; (C) Bcl-2 negative TCCs. Dotted line, topo IIα expression ≥ 10%; solid line, topo IIα expression < 10%.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chao D. T., Korsmeyer S. J. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Dingemans A. M., Witlox M. A., Stallaert R. A., van der Valk P., Postmus P. E., Giaccone G. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999 Aug;5(8):2048–2058. [PubMed] [Google Scholar]
- Holden J. A., Perkins S. L., Snow G. W., Kjeldsberg C. R. Immunohistochemical staining for DNA topoisomerase II in non-Hodgkin's lymphomas. Am J Clin Pathol. 1995 Jul;104(1):54–59. doi: 10.1093/ajcp/104.1.54. [DOI] [PubMed] [Google Scholar]
- Isaacs R. J., Davies S. L., Sandri M. I., Redwood C., Wells N. J., Hickson I. D. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998 Oct 1;1400(1-3):121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Järvinen T. A., Holli K., Kuukasjärvi T., Isola J. J. Predictive value of topoisomerase IIalpha and other prognostic factors for epirubicin chemotherapy in advanced breast cancer. Br J Cancer. 1998 Jun;77(12):2267–2273. doi: 10.1038/bjc.1998.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch B. J., Guinee D. G., Jr, Holden J. A. Human DNA topoisomerase II-alpha: a new marker of cell proliferation in invasive breast cancer. Hum Pathol. 1997 Oct;28(10):1180–1188. doi: 10.1016/s0046-8177(97)90256-2. [DOI] [PubMed] [Google Scholar]
- Mao Y., Yu C., Hsieh T. S., Nitiss J. L., Liu A. A., Wang H., Liu L. F. Mutations of human topoisomerase II alpha affecting multidrug resistance and sensitivity. Biochemistry. 1999 Aug 17;38(33):10793–10800. doi: 10.1021/bi9909804. [DOI] [PubMed] [Google Scholar]
- McPherson J. P., Goldenberg G. J. Induction of apoptosis by deregulated expression of DNA topoisomerase IIalpha. Cancer Res. 1998 Oct 15;58(20):4519–4524. [PubMed] [Google Scholar]
- Monnin K. A., Bronstein I. B., Gaffney D. K., Holden J. A. Elevations of DNA topoisomerase I in transitional cell carcinoma of the urinary bladder: correlation with DNA topoisomerase II-alpha and p53 expression. Hum Pathol. 1999 Apr;30(4):384–391. doi: 10.1016/s0046-8177(99)90112-0. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L., Constantinides C., Papandropoulos J., Theodoropoulos G., Tzonou A., Giannopoulos A., Zervas A., Dimopoulos C. Evaluation of overexpression of p53 tumor suppressor protein in superficial and invasive transitional cell bladder cancer: comparison with DNA ploidy. Urology. 1995 Sep;46(3):334–340. doi: 10.1016/S0090-4295(99)80216-7. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L., Vourlakou C., Zervas A., Tzonou A., Gakiopoulou H., Dimopoulos M. A. The prevalence of bcl-2, p53, and Ki-67 immunoreactivity in transitional cell bladder carcinomas and their clinicopathologic correlates. Hum Pathol. 1998 Feb;29(2):146–154. doi: 10.1016/s0046-8177(98)90225-8. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Sasano H., Yamaki H., Shizawa S., Kikuchi A., Shineha R., Akaishi T., Satomi S., Nagura H. Topoisomerase II alpha expression in esophageal squamous cell carcinoma. Anticancer Res. 1999 May-Jun;19(3A):1873–1880. [PubMed] [Google Scholar]
- Rudolph P., Olsson H., Bonatz G., Ratjen V., Bolte H., Baldetorp B., Fernö M., Parwaresch R., Alm P. Correlation between p53, c-erbB-2, and topoisomerase II alpha expression, DNA ploidy, hormonal receptor status and proliferation in 356 node-negative breast carcinomas: prognostic implications. J Pathol. 1999 Jan;187(2):207–216. doi: 10.1002/(SICI)1096-9896(199901)187:2<207::AID-PATH223>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Wakabayashi T., Yoshida T., Mizuno M., Yoshikawa K., Kikuchi A., Nakashima N., Yoshida J. Immunohistochemical staining of DNA topoisomerase IIalpha in human gliomas. J Neurosurg. 1999 Sep;91(3):477–482. doi: 10.3171/jns.1999.91.3.0477. [DOI] [PubMed] [Google Scholar]
- Tanoguchi K., Sasano H., Yabuki N., Kikuchi A., Ito K., Sato S., Yajima A. Immunohistochemical and two-parameter flow cytometric studies of DNA topoisomerase II alpha in human epithelial ovarian carcinoma and germ cell tumor. Mod Pathol. 1998 Feb;11(2):186–193. [PubMed] [Google Scholar]