Abstract
Aim—To develop a new, simple, and cheap method for estimating antioxidant activity in human fluids.
Methods—The assay measured the capacity of the biological fluids to inhibit the production of thiobarbituric acid reactive substances (TBARS) from sodium benzoate under the influence of the free oxygen radicals derived from Fenton's reaction. A solution of 1 mmol/litre uric acid was used as standard.
Results—The following mean (SD) antioxidative activities were found (as uric acid) in the various biological fluids: serum, 2.04 (0.20) mmol/litre; urine, 176.5 (25.6) µmol/litre; cerebrospinal fluid, 95.0 (26.9) µmol/litre; aqueous humour oculi, 61.25 (9.9) µmol/litre; saliva, 838.5 (48.2) µmol/litre; tears, 247.0 (17.0) µmol/litre; ascites fluid, 270.0 (63.3) µmol/litre; kidney cyst fluid, 387.1 (28.1) µmol/litre. Small samples of the biological material were needed for the analyses: 10 µl of serum and 50–100 µl of other body fluids. In the sera of 48 healthy individuals there was a significant positive correlation between values obtained with the Randox method (as a reference method) and the new method proposed here (correlation coefficient, 0.8728; mean difference between methods, <0.4%).
Conclusions—This method is easy, rapid, reliable, and practical for the routine measurement of total antioxidant activity in serum and other human body fluids. Small samples of biological material are needed for the analyses and the results are comparable with the reference (Randox) method.
Key Words: antioxidant activity • free oxygen radicals • human fluids
Full Text
The Full Text of this article is available as a PDF (165.8 KB).
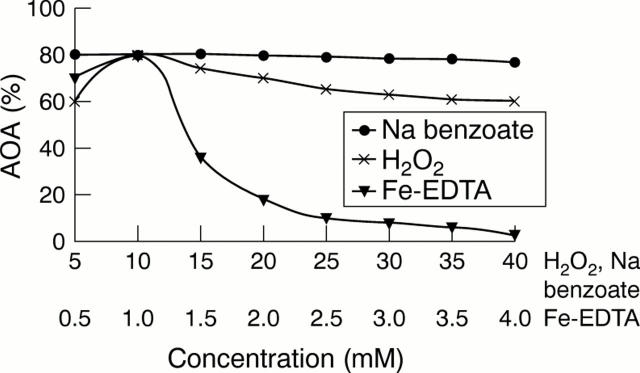
Figure 1 Influence of the concentration of reagents on antioxidant activity (AOA). Na benzoate was used at 5–40 mM (while Fe–EDTA was kept at 1 mM and H2O2 at 10 mM). H2O2 was used at 5–40 mM (while Fe–EDTA was kept at 1 mM and Na benzoate at 10 mM). Fe–EDTA was used at 0.5–4 mM (while H2O2 was kept at 10 mM and Na benzoate at 10 mM).
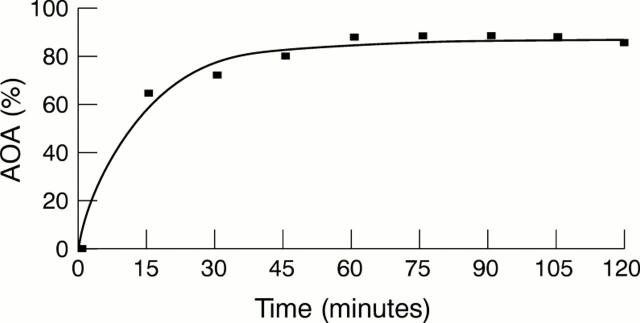
Figure 2 Influence of incubation time on benzoate degradation. AOA, antioxidant activity.
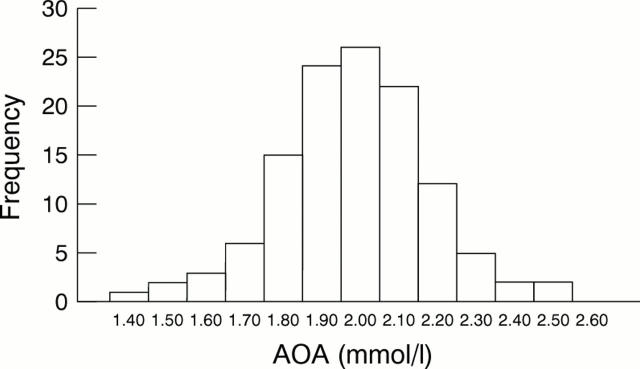
Figure 3 Frequency distribution of serum antioxidant activity (AOA) in healthy subjects.
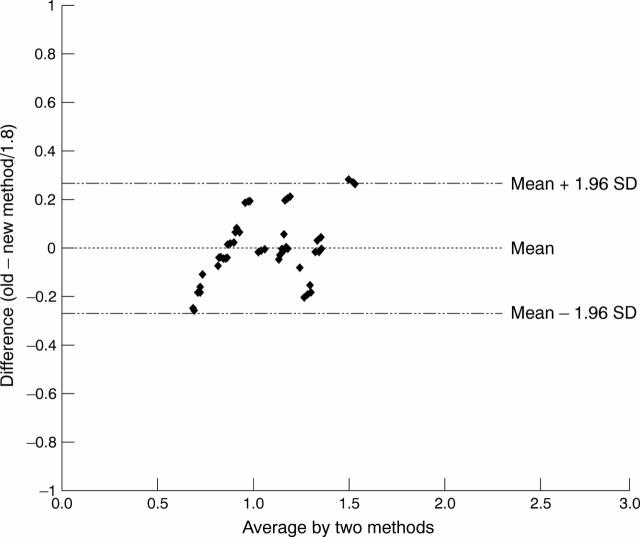
Figure 4 Mean difference between the two methods.
Figure 5 Mean difference between the Randox method and our proposed method after correction.
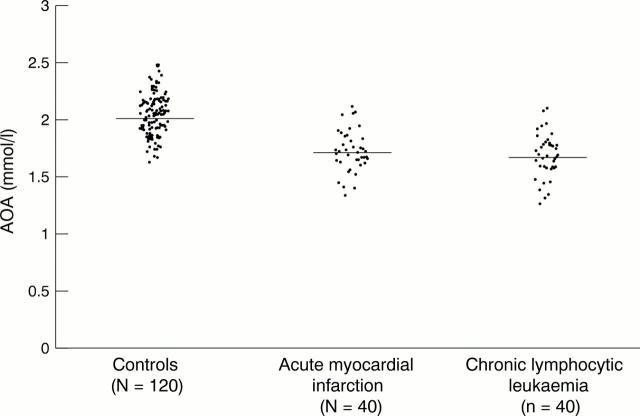
Figure 6 Antioxidant activity (AOA) in human serum.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Davies K. J., Sevanian A., Muakkassah-Kelly S. F., Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986 May 1;235(3):747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G., Young I. S. Effect of anticoagulant choice on apparent antioxidant capacity. Ann Clin Biochem. 1996 Jan;33(Pt 1):93–94. doi: 10.1177/000456329603300121. [DOI] [PubMed] [Google Scholar]
- Goode H. F., Richardson N., Myers D. S., Howdle P. D., Walker B. E., Webster N. R. The effect of anticoagulant choice on apparent total antioxidant capacity using three different methods. Ann Clin Biochem. 1995 Jul;32(Pt 4):413–416. doi: 10.1177/000456329503200410. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of the proteins caeruloplasmin, albumin and transferrin. A study of their activity in serum and synovial fluid from patients with rheumatoid arthritis. Biochim Biophys Acta. 1986 Jan 30;869(2):119–127. doi: 10.1016/0167-4838(86)90286-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Hill C., Blake D. R. Copper stimulated phospholipid membrane peroxidation: antioxidant activity of serum and synovial fluid from patients with rheumatoid arthritis. Clin Chim Acta. 1984 May 16;139(1):85–90. doi: 10.1016/0009-8981(84)90195-5. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Maidt L., Poyer L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem J. 1990 Jul 1;269(1):169–174. doi: 10.1042/bj2690169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidants in human health and disease. Annu Rev Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- Kulikov V. Iu, Molchanova L. V. Opredelenie antiokislitel'noi aktivnosti syvorotki krovi. Lab Delo. 1980;(7):419–421. [PubMed] [Google Scholar]
- Landray M. J., Nuttall S. L., Maxwell S. R., Thorpe G. H. Total antioxidant capacity by enhanced chemiluminescence: contribution of urate. Ann Clin Biochem. 1998 Jul;35(Pt 4):553–554. doi: 10.1177/000456329803500417. [DOI] [PubMed] [Google Scholar]
- Miller N. J. Anticoagulants for total antioxidant activity assay. Ann Clin Biochem. 1996 Jan;33(Pt 1):92–93. doi: 10.1177/000456329603300119. [DOI] [PubMed] [Google Scholar]
- Miller N. J., Johnston J. D., Collis C. S., Rice-Evans C. Serum total antioxidant activity after myocardial infarction. Ann Clin Biochem. 1997 Jan;34(Pt 1):85–90. doi: 10.1177/000456329703400113. [DOI] [PubMed] [Google Scholar]
- Rea C. A., Maxwell S. R., Maslin D. J., Thomason H. L., Thorpe G. H. Anticoagulant effects of antioxidant capacity. Ann Clin Biochem. 1996 Mar;33(Pt 2):174–174. doi: 10.1177/000456329603300218. [DOI] [PubMed] [Google Scholar]
- Reiter R. J. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995 Apr;9(7):526–533. [PubMed] [Google Scholar]
- Rumley A. G., Paterson J. R. Analytical aspects of antioxidants and free radical activity in clinical biochemistry. Ann Clin Biochem. 1998 Mar;35(Pt 2):181–200. doi: 10.1177/000456329803500202. [DOI] [PubMed] [Google Scholar]
- Spektor E. B., Ananenko A. A., Politova L. N. Opredelenie obshchei antiokislitel'noi aktivnosti plazmy krovi i likvora. Lab Delo. 1984;(1):26–28. [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin Sci Mol Med. 1974 Sep;47(3):215–222. doi: 10.1042/cs0470215. [DOI] [PubMed] [Google Scholar]
- Valkonen M., Kuusi T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J Lipid Res. 1997 Apr;38(4):823–833. [PubMed] [Google Scholar]
- Vidláková M., Erazímová J., Horký J., Placer Z. Relationship of serum antioxidative activity to tocopherol and serum inhibitor of lipid peroxidation. Clin Chim Acta. 1972 Jan;36(1):61–66. doi: 10.1016/0009-8981(72)90158-1. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Barclay L. R., Locke S. J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987 Jun 22;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U. The antioxidant efficiency of vitamin C is concentration-dependent. Biochim Biophys Acta. 1986 Oct 29;884(1):119–123. doi: 10.1016/0304-4165(86)90234-5. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford F. P., Whitehead T. P. Is measuring serum antioxidant capacity clinically useful? Ann Clin Biochem. 1998 Jan;35(Pt 1):48–56. doi: 10.1177/000456329803500105. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Piette L. H. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990 Aug 15;265(23):13589–13594. [PubMed] [Google Scholar]
- Yu B. P. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994 Jan;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]