Abstract
Background—The results of immunohistochemical staining are often assessed by semiquantitative scoring. However, these scoring systems are usually non-standardised and there has been little evaluation of the accuracy and reliability of this subjective assessment.
Aims—To assess the accuracy of observer estimation of proportions of objects in an image.
Methods—Images were generated that contained known proportions of pink squares in grids of 50 x 50 and 100 x 100 squares. Observers were shown each image for five seconds in random order and either estimated the proportion of pink squares or selected the image (from a pair of images) that contained the greater proportion of pink squares. The observers were four consultant histopathologists, seven trainee histopathologists, and six control non-histopathologists.
Results—The raw estimations of proportions showed a close correlation with the real proportions, with correlation coefficients of 0.94 and 0.95 for consultant and trainee histopathologists on the 50 x 50 grids. However, the performance in the comparison task was much higher, with an almost perfect classification for grids of equal size even when the proportions only differed by 5%.
Conclusions—Histopathologists can estimate proportions of objects in an image with a reasonable degree of accuracy in this abstract test system. All observers, whether histopathologists or not, can discriminate between proportions that are only 5% different in equal sized image grids. This suggests that the generation and use of carefully calibrated reference images could greatly improve the accuracy and reliability of semiquantitative scoring of immunohistochemical or any other staining.
Key Words: scoring • immunohistochemistry • semiquantitative • interobserver agreement • κ statistics
Full Text
The Full Text of this article is available as a PDF (288.2 KB).
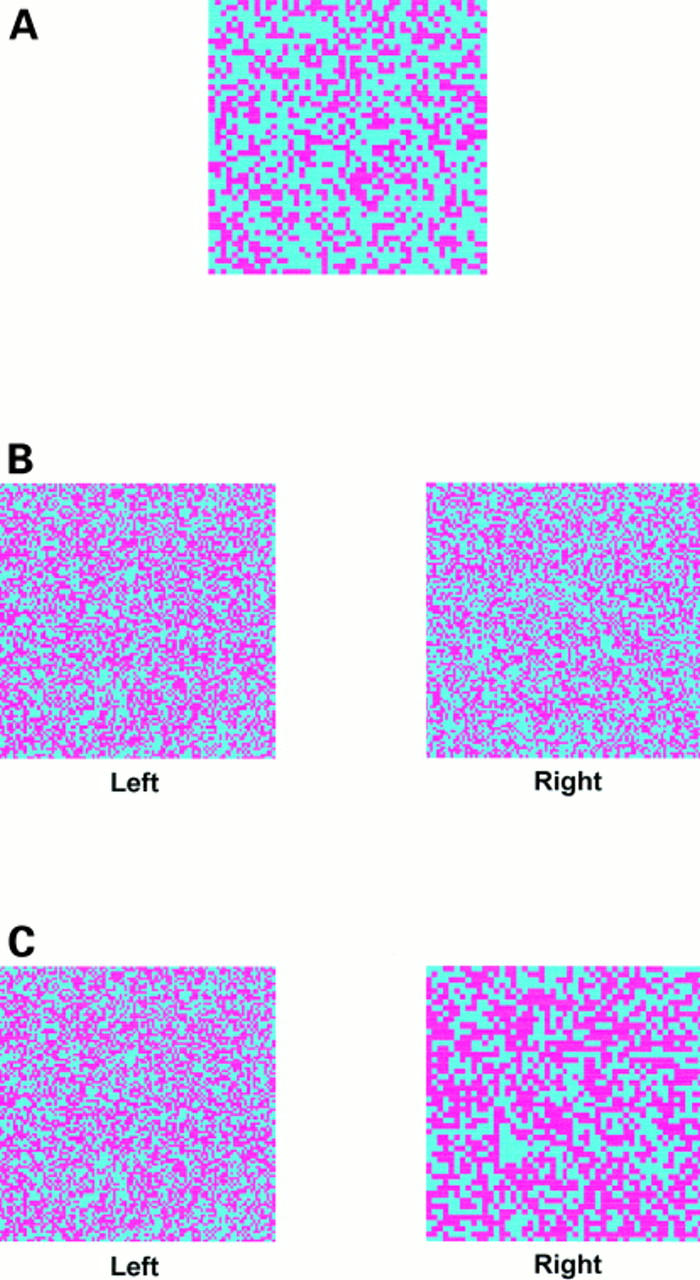
Figure 1 Examples of the test images. (A) A 50 x 50 grid in which 40% of the squares are coloured pink. (B) Two 100 x 100 grids in a comparison image; the left hand grid has 50% pink squares, the right hand grid has 45%. Despite the small difference in the proportion of pink squares between the two grids all the observers correctly selected the left hand grid as the one with the greatest proportion. (C) Two grids in a comparison image. The left hand 100 x 100 grid has 50% pink squares, the right hand 50 x 50 grid has 55% pink squares. Most of the errors made in the entire study related to this image, although 74% of observers still correctly identified the right hand grid as containing the greatest proportion.
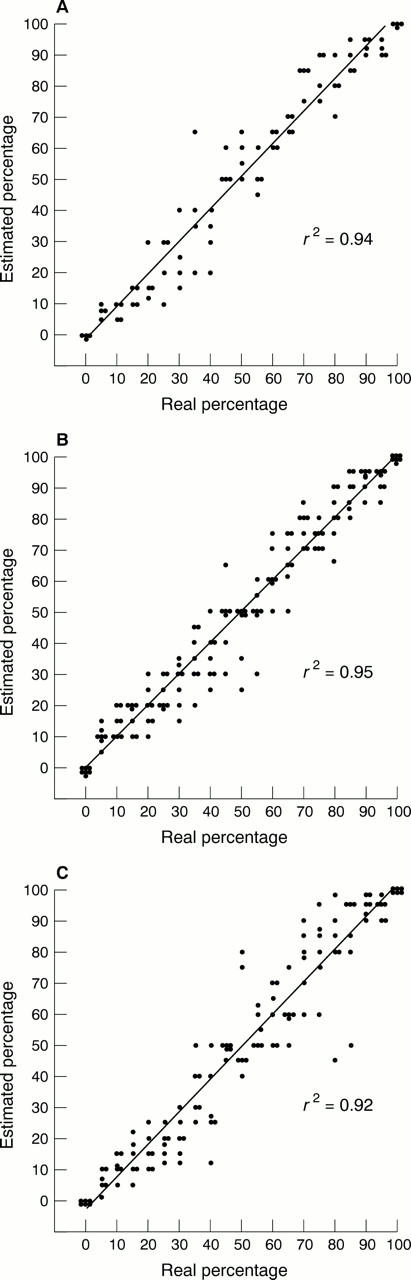
Figure 2 Scattergrams for the estimated proportion of pink squares in a 50 x 50 grid for (A) the consultant histopathologists, (B) the trainee histopathologists, and (C) controls. The regression line and correlation coefficient were derived using the least squares method of regression.

Figure 3 Scattergrams for the estimated proportion of pink squares in a 100 x 100 grid for (A) the consultant histopathologists, (B) the trainee histopathologists, and (C) controls. The regression line and correlation coefficient were derived using the least squares method of regression. It can be seen that the points have a greater scatter around the regression line than for the 50 x 50 grids (fig 2). This scatter is greater in the control group than in the consultant or trainee histopathologist groups and there is a tendency towards overestimation of the proportion.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. M., Millis R. R., Beex L. V., Thorpe S. M., Leake R. E. Increased use of immunohistochemistry for oestrogen receptor measurement in mammary carcinoma: the need for quality assurance. Eur J Cancer. 1998 Oct;34(11):1677–1682. doi: 10.1016/s0959-8049(98)00149-x. [DOI] [PubMed] [Google Scholar]
- Bates A. W., Baithun S. I. Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinicopathological and histological features of 282 cases. Histopathology. 2000 Jan;36(1):32–40. doi: 10.1046/j.1365-2559.2000.00797.x. [DOI] [PubMed] [Google Scholar]
- Bonatz G., Lüttes J., Hamann S., Mettler L., Jonat W., Parwaresch R. Immunohistochemical assessment of p170 provides prognostic information in endometrial carcinoma. Histopathology. 1999 Jan;34(1):43–50. doi: 10.1046/j.1365-2559.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Bànkfalvi A., Terpe H. J., Breukelmann D., Bier B., Rempe D., Pschadka G., Krech R., Lellè R. J., Boecker W. Immunophenotypic and prognostic analysis of E-cadherin and beta-catenin expression during breast carcinogenesis and tumour progression: a comparative study with CD44. Histopathology. 1999 Jan;34(1):25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- Cross S. S., Betmouni S., Burton J. L., Dubé A. K., Feeley K. M., Holbrook M. R., Landers R. J., Lumb P. B., Stephenson T. J. What levels of agreement can be expected between histopathologists assigning cases to discrete nominal categories? A study of the diagnosis of hyperplastic and adenomatous colorectal polyps. Mod Pathol. 2000 Sep;13(9):941–944. doi: 10.1038/modpathol.3880171. [DOI] [PubMed] [Google Scholar]
- Cross S. S. Grading and scoring in histopathology. Histopathology. 1998 Aug;33(2):99–106. doi: 10.1046/j.1365-2559.1998.00495.x. [DOI] [PubMed] [Google Scholar]
- Cross S. S. Kappa statistics as indicators of quality assurance in histopathology and cytopathology. J Clin Pathol. 1996 Jul;49(7):597–599. doi: 10.1136/jcp.49.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Going J. J. Predicting the future: a critical appraisal of cancer prognosis studies. Histopathology. 1999 Dec;35(6):489–494. doi: 10.1046/j.1365-2559.1999.00862.x. [DOI] [PubMed] [Google Scholar]
- Jimenez R. E., Wallis T., Tabasczka P., Visscher D. W. Determination of Her-2/Neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2000 Jan;13(1):37–45. doi: 10.1038/modpathol.3880007. [DOI] [PubMed] [Google Scholar]
- Kaufmann O., Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000 Jan;36(1):8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- Lohmann C. M., League A. A., Clark W. S., Lawson D., DeRose P. B., Cohen C. Bcl-2: bax and bcl-2: Bcl-x ratios by image cytometric quantitation of immunohistochemical expression in ovarian carcinoma: correlation with prognosis. Cytometry. 2000 Feb 15;42(1):61–66. doi: 10.1002/(sici)1097-0320(20000215)42:1<61::aid-cyto9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Matkowskyj K. A., Schonfeld D., Benya R. V. Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem. 2000 Feb;48(2):303–312. doi: 10.1177/002215540004800216. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P., Hamilton P. W., Jasani B. High metallothionein expression is associated with features predictive of aggressive behaviour in endometrial carcinoma. Histopathology. 1999 Jan;34(1):51–55. doi: 10.1046/j.1365-2559.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- McNicol A. M., Richmond J. A. Optimizing immunohistochemistry: antigen retrieval and signal amplification. Histopathology. 1998 Feb;32(2):97–103. doi: 10.1046/j.1365-2559.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- Ordi J., Schammel D. P., Rasekh L., Tavassoli F. A. Sertoliform endometrioid carcinomas of the ovary: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 1999 Oct;12(10):933–940. [PubMed] [Google Scholar]
- Pernick N. L., DaSilva M., Gangi M. D., Crissman J., Adsay V. "Histiocytic markers" in melanoma. Mod Pathol. 1999 Nov;12(11):1072–1077. [PubMed] [Google Scholar]
- Ramsay A. D. Errors in histopathology reporting: detection and avoidance. Histopathology. 1999 Jun;34(6):481–490. doi: 10.1046/j.1365-2559.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Rhodes A., Jasani B., Balaton A. J., Miller K. D. Immunohistochemical demonstration of oestrogen and progesterone receptors: correlation of standards achieved on in house tumours with that achieved on external quality assessment material in over 150 laboratories from 26 countries. J Clin Pathol. 2000 Apr;53(4):292–301. doi: 10.1136/jcp.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes A., Jasani B., Barnes D. M., Bobrow L. G., Miller K. D. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000 Feb;53(2):125–130. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. J., Cross S. S., Harrison R. F. Visualisation of biomedical datasets by use of growing cell structure networks: a novel diagnostic classification technique. Lancet. 1999 Oct 30;354(9189):1518–1521. doi: 10.1016/S0140-6736(99)02186-8. [DOI] [PubMed] [Google Scholar]
- Wang S., Saboorian M. H., Frenkel E., Hynan L., Gokaslan S. T., Ashfaq R. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol. 2000 May;53(5):374–381. doi: 10.1136/jcp.53.5.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn H. C., Burger C. W., van der Valk P., Bonfrèr H. M. Oestrogen, progesterone, and androgen receptors in ovarian neoplasia: correlation between immunohistochemical and biochemical receptor analyses. J Clin Pathol. 2000 Mar;53(3):201–205. doi: 10.1136/jcp.53.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]


