Abstract
Umbilical cord blood (UCB) transplantation is limited to small recipients because of the low haemopoietic cell dose. Children from ethnic minority groups may benefit most from cord blood transplantation. Cohort controlled retrospective data indicate that there is significantly less acute and chronic graft versus host disease associated with the transplantation of human major histocompatibility complex (HLA) identical sibling cord blood compared with HLA identical sibling marrow. Controlled data are not yet available to confirm this observation in unrelated donor cord blood transplantation. The difference in leukaemic relapse seen after cord blood compared with bone marrow transplantation is also unknown. Tentative recommendations for the use of umbilical cord blood for transplantation are as follows. Collection is indicated from healthy newborn siblings when urgent transplantation is required for an older child in a family. The haematologist responsible for the older child, with the approval of the family and the obstetric team, should contact the medical director of the nearest cord blood bank to discuss arrangements for the UCB to be collected and HLA typed. Antenatal blood sampling to HLA type the fetus is not recommended. Umbilical cord blood should be considered when allogeneic transplantation is the treatment of choice for a child who does not have an HLA identical sibling, or a well matched unrelated adult volunteer donor. The potential advantages and disadvantages of using an HLA haplotype matched peripheral blood stem cell family donor rather than an unrelated cord blood donation should be discussed. There are no comparative data available as yet. At present, UCB transplantation should only be considered if a suitably matched donation contains at least 2 x 107/kg nucleated cells. Effectively, this means that most adults and larger children are not suitable recipients.
Key Words: umbilical cord blood transplantation • graft versus host disease • bone marrow transplantation • leukaemia • cell expansion
Full Text
The Full Text of this article is available as a PDF (144.7 KB).

Figure 1 The relation between the infused CD34+ cell dose and (A) platelet and (B) neutrophil recovery after unrelated umbilical cord blood transplantation. The numbers within the graphs refer to the dose of CD34+ cells (x106/kg). There is a strong direct correlation between cell dose and peripheral blood recovery. Transplants containing less than 0.3 x 106/kg CD34+ cells have a high probability of poor engraftment or non-engraftment.

Figure 2 The effect of CD34+ cell dose on survival after unrelated umbilical cord blood transplantation. (A) There is a direct correlation between increasing cell dose and the probability of survival at one year post transplant. Patients receiving less than 0.3 x 106/kg CD34+ cells have a significantly higher probability of death in the first year after transplantation than those receiving a higher CD34+ cell dose. The numbers within the graphs refer to the dose of CD34+ cells (x106/kg). (B) The relatively minor effect of human major histocompatibility complex (HLA) mismatch on survival compared with the major effect of cell dose. HLA typing was performed by intermediate resolution DNA methodology. mm, number of HLA loci mismatched.
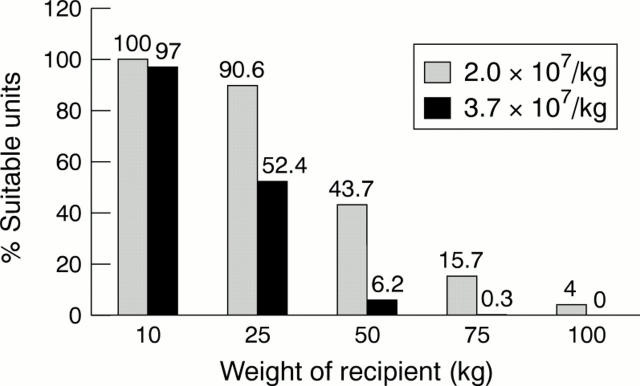
Figure 3 The proportion of recipients of different weight for whom an optimal cord blood donation (> 3.7 x 107/kg nucleated cells) or a just sufficient donation (> 2.0 x 107/kg) of nucleated blood cells is available in the Bristol cord blood bank. With the exception of when the potential recipient has a common HLA type, the chance of finding a large enough donation for an adult with two or less human major histocompatibility complex (HLA) mismatches using high resolution DNA typing is very low.
Figure 4 Engraftment of human cord blood cells with or without in vitro expansion in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice after five weeks. Cord blood CD34+ cells were cultured in vitro for three, seven, or 10 days in stem cell factor (SCF), Flt3 ligand (Flt3), thrombopoietin (TPO), interleukin 3 (IL-3), IL-6, and granulocyte colony stimulating factor (G-CSF), all at 10 ng/ml. Seventy six mice received fresh or cultured cells from eight different cord bloods. (A) SRC are still present after three, seven, and 10 days of culture. The mean level of engraftment with cultured cells was lower than with fresh cells (1% v 7.4%) when the same number of expanded or fresh CD34+ cells was transplanted. This suggests that although engraftment of expanded cells occurs there is less efficient proliferation and differentiation in vivo, resulting in a lower proportion of human cells in the bone marrow after five weeks. (B) If the input number of expanded cells is reduced, engraftment is seen with the expanded progeny of only 0.6 x 104 CD34+ cells. This suggests that some expansion of SRC numbers may occur in vitro.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcese W., Iori A. P., Screnci M., Guglielmi C., Mengarelli A., Carmini D., Testi A. M., Moleti M. L., Cimino G., Perrone P. Umbilical cord blood transplant from HLA-mismatched unrelated donor in high-risk leukemia. Bone Marrow Transplant. 1998 Jun;21 (Suppl 3):S85–S86. [PubMed] [Google Scholar]
- Armitage S., Fehily D., Dickinson A., Chapman C., Navarrete C., Contreras M. Cord blood banking: volume reduction of cord blood units using a semi-automated closed system. Bone Marrow Transplant. 1999 Mar;23(5):505–509. doi: 10.1038/sj.bmt.1701591. [DOI] [PubMed] [Google Scholar]
- Armitage S., Warwick R., Fehily D., Navarrete C., Contreras M. Cord blood banking in London: the first 1000 collections. Bone Marrow Transplant. 1999 Jul;24(2):139–145. doi: 10.1038/sj.bmt.1701881. [DOI] [PubMed] [Google Scholar]
- Aversa F., Tabilio A., Terenzi A., Velardi A., Falzetti F., Giannoni C., Iacucci R., Zei T., Martelli M. P., Gambelunghe C. Successful engraftment of T-cell-depleted haploidentical "three-loci" incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994 Dec 1;84(11):3948–3955. [PubMed] [Google Scholar]
- Bahçeci E., Read E. J., Leitman S., Childs R., Dunbar C., Young N. S., Barrett A. J. CD34+ cell dose predicts relapse and survival after T-cell-depleted HLA-identical haematopoietic stem cell transplantation (HSCT) for haematological malignancies. Br J Haematol. 2000 Feb;108(2):408–414. doi: 10.1046/j.1365-2141.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Wang J. C., Kapp U., Bonnet D., Dick J. E. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Douglas G. W., Hangoc G., Cooper S., Bard J., English D., Arny M., Thomas L., Boyse E. A. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M. S., Wagner J. E. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997 Dec 15;90(12):4665–4678. [PubMed] [Google Scholar]
- Deacock S. J., Schwarer A. P., Bridge J., Batchelor J. R., Goldman J. M., Lechler R. I. Evidence that umbilical cord blood contains a higher frequency of HLA class II-specific alloreactive T cells than adult peripheral blood. A limiting dilution analysis. Transplantation. 1992 May;53(5):1128–1134. doi: 10.1097/00007890-199205000-00028. [DOI] [PubMed] [Google Scholar]
- Denning-Kendall P. A., Nicol A., Horsley H., Donaldson C., Bradley B., Hows J. M. Is in vitro expansion of human cord blood cells clinically relevant? Bone Marrow Transplant. 1998 Feb;21(3):225–232. doi: 10.1038/sj.bmt.1701078. [DOI] [PubMed] [Google Scholar]
- Dick J. E. Absence of CD34 on some human SCID-repopulating cells. Ann N Y Acad Sci. 1999 Apr 30;872:211–219. doi: 10.1111/j.1749-6632.1999.tb08466.x. [DOI] [PubMed] [Google Scholar]
- Donaldson C., Armitage W. J., Denning-Kendall P. A., Nicol A. J., Bradley B. A., Hows J. M. Optimal cryopreservation of human umbilical cord blood. Bone Marrow Transplant. 1996 Oct;18(4):725–731. [PubMed] [Google Scholar]
- Donaldson C., Armitage W. J., Laundy V., Barron C., Buchanan R., Webster J., Bradley B., Hows J. Impact of obstetric factors on cord blood donation for transplantation. Br J Haematol. 1999 Jul;106(1):128–132. doi: 10.1046/j.1365-2141.1999.01507.x. [DOI] [PubMed] [Google Scholar]
- Donaldson C., Buchanan R., Webster J., Laundy V., Horsley H., Barron C., Anderson N., Bradley B., Hows J. Development of a district Cord Blood Bank: a model for cord blood banking in the National Health Service. Bone Marrow Transplant. 2000 Apr;25(8):899–905. doi: 10.1038/sj.bmt.1702332. [DOI] [PubMed] [Google Scholar]
- Gluckman E., Broxmeyer H. A., Auerbach A. D., Friedman H. S., Douglas G. W., Devergie A., Esperou H., Thierry D., Socie G., Lehn P. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989 Oct 26;321(17):1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- Gluckman E., Rocha V., Boyer-Chammard A., Locatelli F., Arcese W., Pasquini R., Ortega J., Souillet G., Ferreira E., Laporte J. P. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997 Aug 7;337(6):373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- Hagihara M., Chargui J., Gansuvd B., Tsuchida F., Sato T., Hotta T., Kato S. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. 1999 Dec;24(11):1229–1233. doi: 10.1038/sj.bmt.1702050. [DOI] [PubMed] [Google Scholar]
- Haque K., Truman C., Dittmer I., Denning-Kendall P., Hows J., Bradley B. Frequencies of cytotoxic T lymphocyte precursor estimate in three different populations. Asian Pac J Allergy Immunol. 1999 Jun;17(2):93–99. [PubMed] [Google Scholar]
- Harris D. T., Schumacher M. J., Locascio J., Besencon F. J., Olson G. B., DeLuca D., Shenker L., Bard J., Boyse E. A. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hows J. M., Bradley B. A., Marsh J. C., Luft T., Coutinho L., Testa N. G., Dexter T. M. Growth of human umbilical-cord blood in longterm haemopoietic cultures. Lancet. 1992 Jul 11;340(8811):73–76. doi: 10.1016/0140-6736(92)90396-k. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Laughlin M., Graham M. L., Smith C., Olson J. F., Halperin E. C., Ciocci G., Carrier C., Stevens C. E., Rubinstein P. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996 Jul 18;335(3):157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- Lapidot T., Pflumio F., Doedens M., Murdoch B., Williams D. E., Dick J. E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992 Feb 28;255(5048):1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- Larochelle A., Vormoor J., Hanenberg H., Wang J. C., Bhatia M., Lapidot T., Moritz T., Murdoch B., Xiao X. L., Kato I. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996 Dec;2(12):1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- Larochelle A., Vormoor J., Lapidot T., Sher G., Furukawa T., Li Q., Shultz L. D., Olivieri N. F., Stamatoyannopoulos G., Dick J. E. Engraftment of immune-deficient mice with primitive hematopoietic cells from beta-thalassemia and sickle cell anemia patients: implications for evaluating human gene therapy protocols. Hum Mol Genet. 1995 Feb;4(2):163–172. doi: 10.1093/hmg/4.2.163. [DOI] [PubMed] [Google Scholar]
- Laughlin M. J., Rizzieri D. A., Smith C. A., Moore J. O., Lilly S., McGaughey D., Martin P., Carrier C., Stevens C. E., Rubinstein P. Hematologic engraftment and reconstitution of immune function post unrelated placental cord blood transplant in an adult with acute lymphocytic leukemia. Leuk Res. 1998 Mar;22(3):215–219. doi: 10.1016/s0145-2126(97)00171-9. [DOI] [PubMed] [Google Scholar]
- Pecora A. L., Stiff P., Jennis A., Goldberg S., Rosenbluth R., Price P., Goltry K. L., Douville J., Armstrong R. D., Smith A. K. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant. 2000 Apr;25(7):797–799. doi: 10.1038/sj.bmt.1702222. [DOI] [PubMed] [Google Scholar]
- Risdon G., Gaddy J., Horie M., Broxmeyer H. E. Alloantigen priming induces a state of unresponsiveness in human umbilical cord blood T cells. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2413–2417. doi: 10.1073/pnas.92.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V., Wagner J. E., Jr, Sobocinski K. A., Klein J. P., Zhang M. J., Horowitz M. M., Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000 Jun 22;342(25):1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- Roncarolo M. G., Bigler M., Martino S., Ciuti E., Tovo P. A., Wagner J. Immune functions of cord blood cells before and after transplantation. J Hematother. 1996 Apr;5(2):157–160. doi: 10.1089/scd.1.1996.5.157. [DOI] [PubMed] [Google Scholar]
- Shen B. J. [Human umbilical cord blood transplantation in 4 cases with advanced solid tumors]. Zhonghua Zhong Liu Za Zhi. 1993 Mar;15(2):152–154. [PubMed] [Google Scholar]
- Vormoor J., Lapidot T., Pflumio F., Risdon G., Patterson B., Broxmeyer H. E., Dick J. E. SCID mice as an in vivo model of human cord blood hematopoiesis. Blood Cells. 1994;20(2-3):316–322. [PubMed] [Google Scholar]
- Wagner J. E., Broxmeyer H. E., Byrd R. L., Zehnbauer B., Schmeckpeper B., Shah N., Griffin C., Emanuel P. D., Zuckerman K. S., Cooper S. Transplantation of umbilical cord blood after myeloablative therapy: analysis of engraftment. Blood. 1992 Apr 1;79(7):1874–1881. [PubMed] [Google Scholar]
- Wagner J. E., Kernan N. A., Steinbuch M., Broxmeyer H. E., Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995 Jul 22;346(8969):214–219. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- Wagner J. E., Rosenthal J., Sweetman R., Shu X. O., Davies S. M., Ramsay N. K., McGlave P. B., Sender L., Cairo M. S. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood. 1996 Aug 1;88(3):795–802. [PubMed] [Google Scholar]
- Wang J. C., Doedens M., Dick J. E. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997 Jun 1;89(11):3919–3924. [PubMed] [Google Scholar]
- Weinreb S., Delgado J. C., Clavijo O. P., Yunis E. J., Bayer-Zwirello L., Polansky L., Deluhery L., Cohn G., Yao J. T., Stec T. C. Transplantation of unrelated cord blood cells. Bone Marrow Transplant. 1998 Jul;22(2):193–196. doi: 10.1038/sj.bmt.1701309. [DOI] [PubMed] [Google Scholar]
- van der Loo J. C., Ploemacher R. E. Marrow- and spleen-seeding efficiencies of all murine hematopoietic stem cell subsets are decreased by preincubation with hematopoietic growth factors. Blood. 1995 May 1;85(9):2598–2606. [PubMed] [Google Scholar]