Abstract
Aim—To assess the value of immunohistochemistry in discriminating between BRCA1 associated and non-BRCA1 associated breast tumours.
Methods—Four commercially available anti-BRCA1 antibodies were used on 45 paraffin wax embedded tumoral samples from patients with (seven of 45) and without (38 of 45) BRCA1 germline mutations. In all patients, the BRCA1 gene had been studied previously by means of the protein truncation test (PTT), conformational sensitive gel electrophoresis (CSGE), and direct sequencing of genomic DNA. Immunohistochemistry was carried out using the standard avidin–biotin immunoperoxidase method. Antigen retrieval was carried out by means of microwave pretreatment or autoclaving. The antibody panel used comprised D-20 (1/500), I-20 (1/100), K-18 (1/100), and MS110 (Ab-1; 1/50).
Results—No immunohistochemical differences in BRCA1 protein expression were found between cases with and without BRCA1 germline mutations. All positive cases showed predominantly cytoplasmic staining, in both tumoral and non-tumoral cells, with the polyclonal antibodies D-20, I-20, and K-18. After heating pretreatment both nuclear and cytoplasmic staining were found in tumoral and non-tumoral cells with the I-20 antibody. Only the monoclonal antibody MS110 showed a predominantly nuclear staining after microwave oven treatment.
Conclusions—Commercially available BRCA1 antibodies lack the specificity required to identify the BRCA1 protein and thus are not useful for establishing differences between familial and sporadic breast tumours, or between BRCA1 associated and non-BRCA1 associated breast tumours.
Key Words: BRCA1 • breast carcinoma • immunohistochemical study
Full Text
The Full Text of this article is available as a PDF (184.8 KB).
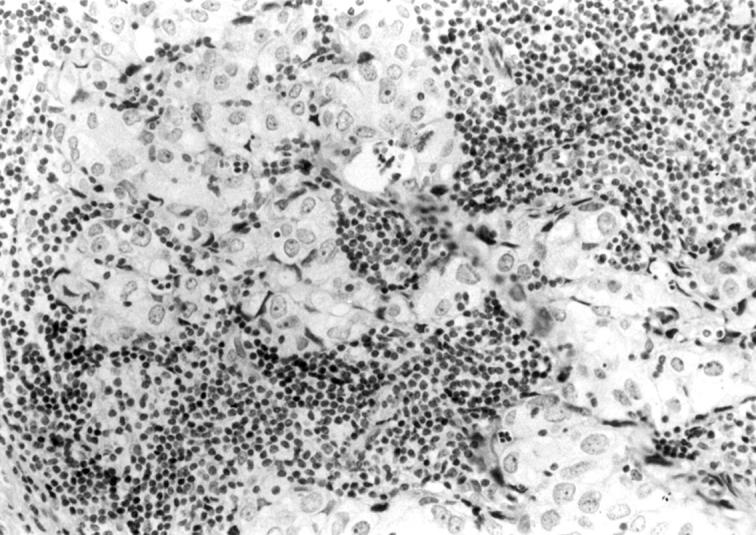
Figure 1 Ab-1 negative staining in a familial breast cancer from a patient without BRCA1 mutations. Note the positive staining in stromal lymphocytes surrounding tumoral cells.
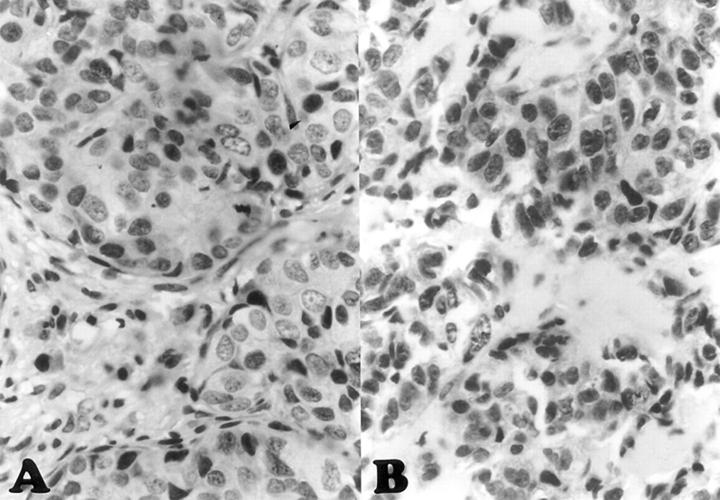
Figure 2 Ab-1 nuclear staining in familial breast cancer cases from patients (A) with and (B) without BRCA1 germline mutations.
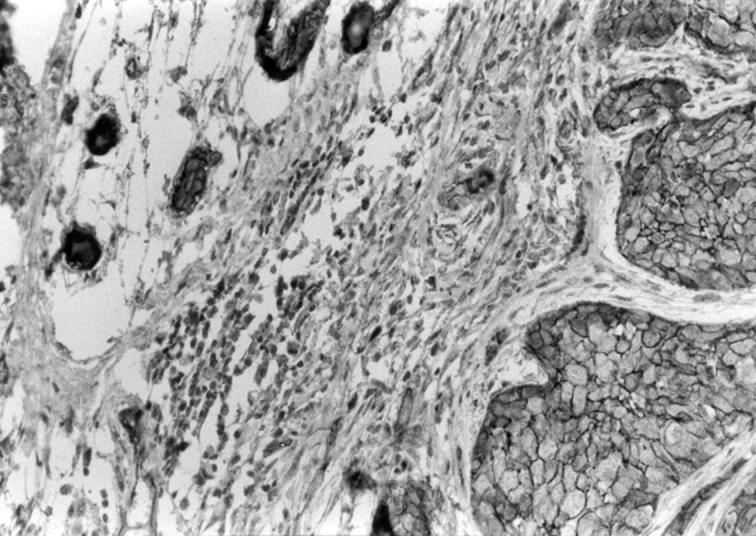
Figure 3 Membrane staining pattern with the D-20 antibody in a familial breast cancer from a patient with BRCA1 mutation.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard-Gallon D. J., Crespin N. C., Maurizis J. C., Bignon Y. J. Cross-reaction between antibodies raised against the last 20 C-terminal amino acids of BRCA 1 (C-20) and human EGF and EGF-R in MCF 10a human mammary epithelial cell line. Int J Cancer. 1997 Mar 28;71(1):123–126. doi: 10.1002/(sici)1097-0215(19970328)71:1<123::aid-ijc20>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bernard-Gallon D. J., De Latour M. P., Rio P. G., Penault-Llorca F. M., Favy D. A., Hizel C., Chassagne J., Bignon Y. J. Subcellular localization of BRCA1 protein in sporadic breast carcinoma with or without allelic loss of BRCA1 gene. Int J Oncol. 1999 Apr;14(4):653–661. doi: 10.3892/ijo.14.4.653. [DOI] [PubMed] [Google Scholar]
- Bièche I., Noguès C., Lidereau R. Overexpression of BRCA2 gene in sporadic breast tumours. Oncogene. 1999 Sep 16;18(37):5232–5238. doi: 10.1038/sj.onc.1202903. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Verma I. M. Transcriptional activation by BRCA1. Nature. 1996 Aug 22;382(6593):678–679. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen C. F., Riley D. J., Allred D. C., Chen P. L., Von Hoff D., Osborne C. K., Lee W. H. Aberrant subcellular localization of BRCA1 in breast cancer. Science. 1995 Nov 3;270(5237):789–791. doi: 10.1126/science.270.5237.789. [DOI] [PubMed] [Google Scholar]
- Coene E., Van Oostveldt P., Willems K., van Emmelo J., De Potter C. R. BRCA1 is localized in cytoplasmic tube-like invaginations in the nucleus. Nat Genet. 1997 Jun;16(2):122–124. doi: 10.1038/ng0697-122. [DOI] [PubMed] [Google Scholar]
- Cortez D., Wang Y., Qin J., Elledge S. J. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999 Nov 5;286(5442):1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Fan S., Wang J., Yuan R., Ma Y., Meng Q., Erdos M. R., Pestell R. G., Yuan F., Auborn K. J., Goldberg I. D. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999 May 21;284(5418):1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- Frierson H. F., Jr, Wolber R. A., Berean K. W., Franquemont D. W., Gaffey M. J., Boyd J. C., Wilbur D. C. Interobserver reproducibility of the Nottingham modification of the Bloom and Richardson histologic grading scheme for infiltrating ductal carcinoma. Am J Clin Pathol. 1995 Feb;103(2):195–198. doi: 10.1093/ajcp/103.2.195. [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Silva J. M., Dominguez G., Garcia J. M., Martinez G., Vargas J., Provencio M., España P., Bonilla F. Detection of loss of heterozygosity at RAD51, RAD52, RAD54 and BRCA1 and BRCA2 loci in breast cancer: pathological correlations. Br J Cancer. 1999 Oct;81(3):503–509. doi: 10.1038/sj.bjc.6690722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen L. C., Avrutskaya A. V., Latour A. M., Koller B. H., Leadon S. A. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998 Aug 14;281(5379):1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- Gudas J. M., Nguyen H., Li T., Cowan K. H. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995 Oct 15;55(20):4561–4565. [PubMed] [Google Scholar]
- Hartmann L. C., Schaid D. J., Woods J. E., Crotty T. P., Myers J. L., Arnold P. G., Petty P. M., Sellers T. A., Johnson J. L., McDonnell S. K. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999 Jan 14;340(2):77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Thompson M. E., Szabo C., Robinson-Benion C., Arteaga C. L., King M. C., Jensen R. A. Growth retardation and tumour inhibition by BRCA1. Nat Genet. 1996 Mar;12(3):298–302. doi: 10.1038/ng0396-298. [DOI] [PubMed] [Google Scholar]
- Jarvis E. M., Kirk J. A., Clarke C. L. Loss of nuclear BRCA1 expression in breast cancers is associated with a highly proliferative tumor phenotype. Cancer Genet Cytogenet. 1998 Mar;101(2):109–115. doi: 10.1016/s0165-4608(97)00267-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Thompson M. E., Jetton T. L., Szabo C. I., van der Meer R., Helou B., Tronick S. R., Page D. L., King M. C., Holt J. T. BRCA1 is secreted and exhibits properties of a granin. Nat Genet. 1996 Mar;12(3):303–308. doi: 10.1038/ng0396-303. [DOI] [PubMed] [Google Scholar]
- Kainu T., Kononen J., Johansson O., Olsson H., Borg A, Isola J. Detection of germline BRCA1 mutations in breast cancer patients by quantitative messenger RNA in situ hybridization. Cancer Res. 1996 Jul 1;56(13):2912–2915. [PubMed] [Google Scholar]
- Koonin E. V., Altschul S. F., Bork P. BRCA1 protein products ... Functional motifs... Nat Genet. 1996 Jul;13(3):266–268. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- Kote-Jarai Z., Eeles R. A. BRCA1, BRCA2 and their possible function in DNA damage response. Br J Cancer. 1999 Dec;81(7):1099–1102. doi: 10.1038/sj.bjc.6690814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. Y., Jin Y. T., Chang T. W., Lin P. W., Su I. J. Immunolocalization of BRCA1 protein in normal breast tissue and sporadic invasive ductal carcinomas: a correlation with other biological parameters. Histopathology. 1999 Feb;34(2):106–112. doi: 10.1046/j.1365-2559.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Monteiro A. N., August A., Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):13595–13599. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Shao N., Ahmad M., Reddy E. S. Antisense RNA to the putative tumor suppressor gene BRCA1 transforms mouse fibroblasts. Oncogene. 1996 Feb 1;12(3):523–528. [PubMed] [Google Scholar]
- Ribieras S., Magdinier F., Leclerc D., Lenoir G., Frappart L., Dante R. Abundance of BRCA1 transcripts in human cancer and lymphoblastoid cell lines carrying BRCA1 germ-line alterations. Int J Cancer. 1997 Nov 27;73(5):715–718. doi: 10.1002/(sici)1097-0215(19971127)73:5<715::aid-ijc17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rio P. G., Maurizis J. C., Peffault de Latour M., Bignon Y. J., Bernard-Gallon D. J. Quantification of BRCA1 protein in sporadic breast carcinoma with or without loss of heterozygosity of the BRCA1 gene. Int J Cancer. 1999 Mar 15;80(6):823–826. doi: 10.1002/(sici)1097-0215(19990315)80:6<823::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Scully R., Ganesan S., Brown M., De Caprio J. A., Cannistra S. A., Feunteun J., Schnitt S., Livingston D. M. Location of BRCA1 in human breast and ovarian cancer cells. Science. 1996 Apr 5;272(5258):123–126. doi: 10.1126/science.272.5258.123. [DOI] [PubMed] [Google Scholar]
- Shao N., Chai Y. L., Shyam E., Reddy P., Rao V. N. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene. 1996 Jul 4;13(1):1–7. [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Evans D. G., Ponder B. A. Allele losses in the region 17q12-21 in familial breast and ovarian cancer involve the wild-type chromosome. Nat Genet. 1992 Oct;2(2):128–131. doi: 10.1038/ng1092-128. [DOI] [PubMed] [Google Scholar]
- Taylor J., Lymboura M., Pace P. E., A'hern R. P., Desai A. J., Shousha S., Coombes R. C., Ali S. An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. Int J Cancer. 1998 Aug 21;79(4):334–342. doi: 10.1002/(sici)1097-0215(19980821)79:4<334::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Thakur S., Zhang H. B., Peng Y., Le H., Carroll B., Ward T., Yao J., Farid L. M., Couch F. J., Wilson R. B. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997 Jan;17(1):444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., Payton M. N., Elliott G. S., Buaas F. W., Cajulis E. E., Grosshans D., Ramos L., Reese D. M., Slamon D. J., Calzone F. J. Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta11b. Oncogene. 1997 Jan 9;14(1):1–16. doi: 10.1038/sj.onc.1200924. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., Payton M. N., Pekar S. K., Zhang K., Pacifici R. E., Gudas J. L., Thukral S., Calzone F. J., Reese D. M., Slamon D. I. BRCA1 protein products: antibody specificity... Nat Genet. 1996 Jul;13(3):264–265. doi: 10.1038/ng0796-264. [DOI] [PubMed] [Google Scholar]
- Wilson C. A., Ramos L., Villaseñor M. R., Anders K. H., Press M. F., Clarke K., Karlan B., Chen J. J., Scully R., Livingston D. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999 Feb;21(2):236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Honda K., Inamoto T., Shinohara H., Yamauchi A., Suga K., Okuyama T., Shimada T., Kodama H., Noguchi S. Reduction of BRCA1 protein expression in Japanese sporadic breast carcinomas and its frequent loss in BRCA1-associated cases. Clin Cancer Res. 1999 Jun;5(6):1249–1261. [PubMed] [Google Scholar]