Abstract
Aims—To investigate whether the three different AP-2 isoforms are expressed differently in colorectal adenomas and carcinomas.
Methods—The study comprised 43 randomly selected patients diagnosed and treated at Kuopio University Hospital in 1996 for colorectal adenocarcinoma (n = 30) and colorectal adenoma (n = 13). The expression of AP-2α, AP-2ß, and AP-2γ was analysed by immunohistochemistry (IHC) and the mRNA status of AP-2α was determined by in situ hybridisation (ISH) and confirmed by reverse transcription polymerase chain reaction (RT-PCR). AP-2 expression patterns were correlated with clinicopathological variables.
Results—In adenomas and carcinomas, AP-2ß cytoplasmic positivity was higher than that of AP-2α or AP-2γ. AP-2α expression was reduced in advanced Dukes's stage carcinomas. In high grade carcinomas, both AP-2α and AP-2γ expression was reduced. ISH demonstrated increased AP-2α values in high grade carcinomas. Seven of 30 carcinoma specimens displayed a moderate or strong mRNA signal, despite being negative for AP-2α protein. RT-PCR from AP-2α mRNA and protein positive tumours confirmed that the positive signal in ISH originated from the exon 2 of TFAP2A.
Conclusions—AP-2α was reduced in advanced Dukes's stage adenocarcinomas. Together with reduced AP-2γ expression in high grade carcinomas, this might contribute to tumour progression. The discrepancy between mRNA and protein expression suggests that post-transcriptional regulatory mechanisms might modify the availability of functional AP-2α protein in colorectal carcinoma.
Key Words: AP-2 proteins • immunohistochemistry • in situ hybridisation • colorectal neoplasms
Full Text
The Full Text of this article is available as a PDF (249.9 KB).
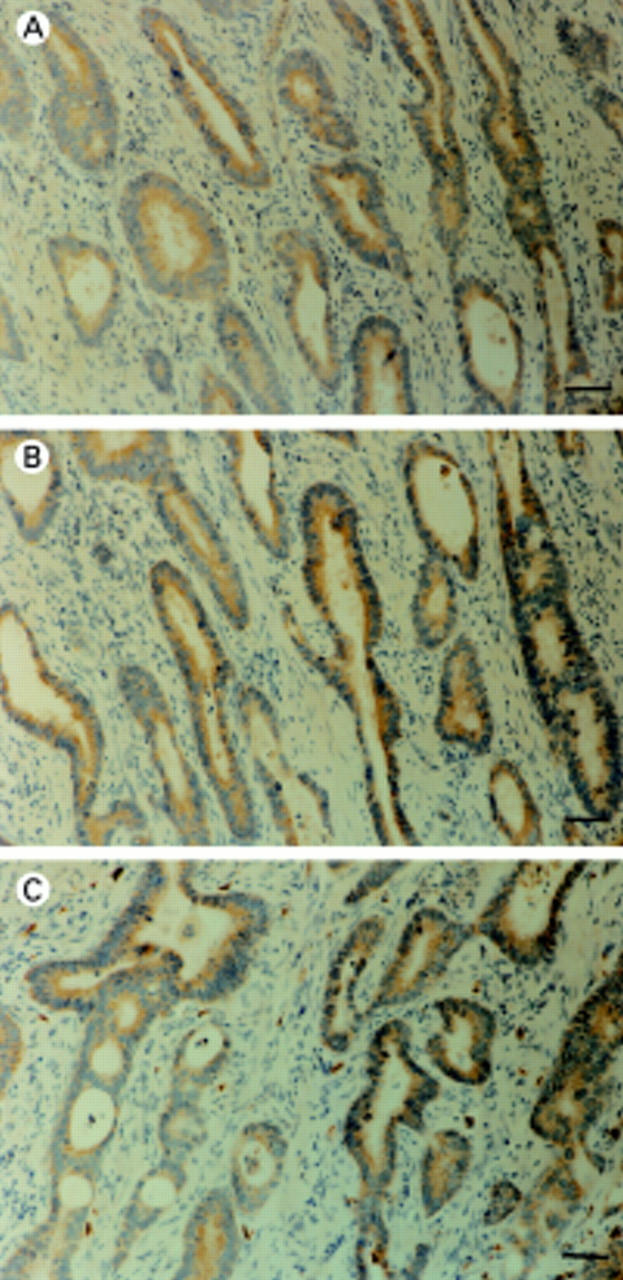
Figure 1 AP-2 immunohistochemical analysis: diffuse cytoplasmic (A) AP-2α, (B) AP-2ß, and (C) AP-2γ expression in colorectal adenocarcinoma cells. Bar, 60 µm.
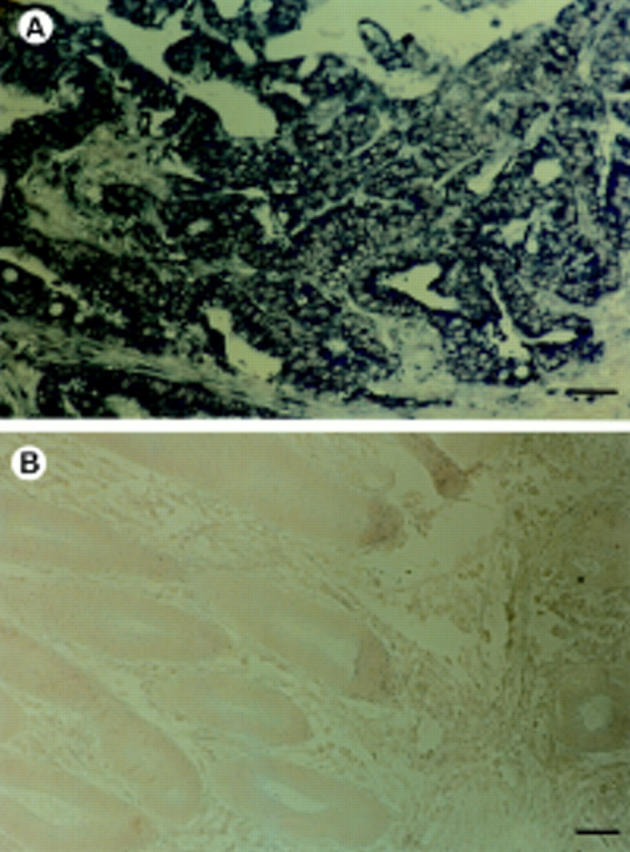
Figure 2 (A) mRNA in situ hybridisation analysis showing strong diffuse (+++) AP-2α expression in colorectal adenocarcinoma and (B) negative control. Bar, 60 µm.
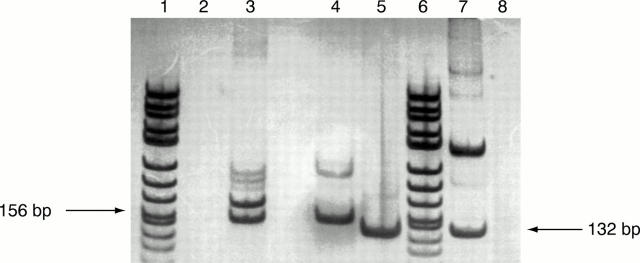
Figure 3 Reverse transcription polymerase chain reaction (RT-PCR) analysis of AP-2α expression was performed on the following extracts: lanes 1 and 6, x174 DNA/Hin FI marker (0.4 µg); lane 2, a negative PCR control for ß actin in which the template was replaced by water; lane 3, a positive control for ß actin RNA (from Clontech human placenta cDNA library); lane 4, case number 28 contains a PCR product of the same molecular size (156 bp; ß actin) as the positive control sample; lane 5, case number 28 contains a PCR product of the same molecular size (132 bp; AP-2α) as the positive control sample; lane 7, positive control sample (from HeLa cDNA; AP-2α); lane 8, a negative PCR control for AP-2α in which the template was replaced by water.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttila M. A., Kellokoski J. K., Moisio K. I., Mitchell P. J., Saarikoski S., Syrjänen K., Kosma V. M. Expression of transcription factor AP-2alpha predicts survival in epithelial ovarian cancer. Br J Cancer. 2000 Jun;82(12):1974–1983. doi: 10.1054/bjoc.2000.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Eli M. Molecular mechanisms of melanoma metastasis. J Cell Physiol. 1997 Nov;173(2):275–278. doi: 10.1002/(SICI)1097-4652(199711)173:2<275::AID-JCP35>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bosari S., Viale G., Bossi P., Maggioni M., Coggi G., Murray J. J., Lee A. K. Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J Natl Cancer Inst. 1994 May 4;86(9):681–687. doi: 10.1093/jnci/86.9.681. [DOI] [PubMed] [Google Scholar]
- Bosher J. M., Williams T., Hurst H. C. The developmentally regulated transcription factor AP-2 is involved in c-erbB-2 overexpression in human mammary carcinoma. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):744–747. doi: 10.1073/pnas.92.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Kannan P., Imhof A., Bauer R., Yim S. O., Glockshuber R., Van Dyke M. W., Tainsky M. A. An alternatively spliced mRNA from the AP-2 gene encodes a negative regulator of transcriptional activation by AP-2. Mol Cell Biol. 1993 Jul;13(7):4174–4185. doi: 10.1128/mcb.13.7.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen C. F., Riley D. J., Allred D. C., Chen P. L., Von Hoff D., Osborne C. K., Lee W. H. Aberrant subcellular localization of BRCA1 in breast cancer. Science. 1995 Nov 3;270(5237):789–791. doi: 10.1126/science.270.5237.789. [DOI] [PubMed] [Google Scholar]
- Day D. A., Tuite M. F. Post-transcriptional gene regulatory mechanisms in eukaryotes: an overview. J Endocrinol. 1998 Jun;157(3):361–371. doi: 10.1677/joe.0.1570361. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Ragoussis J., Stamp G. W., Allan G. J., Trowsdale J. Frequent loss of heterozygosity on chromosome 6 in human ovarian carcinoma. Br J Cancer. 1993 Mar;67(3):551–559. doi: 10.1038/bjc.1993.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Muchardt C., Xia Y. R., Klisak I., Mohandas T., Sparkes R. S., Lusis A. J. Localization of the gene for the DNA-binding protein AP-2 to human chromosome 6p22.3-pter. Genomics. 1991 Aug;10(4):1100–1102. doi: 10.1016/0888-7543(91)90209-w. [DOI] [PubMed] [Google Scholar]
- Gee J. M., Robertson J. F., Ellis I. O., Nicholson R. I., Hurst H. C. Immunohistochemical analysis reveals a tumour suppressor-like role for the transcription factor AP-2 in invasive breast cancer. J Pathol. 1999 Dec;189(4):514–520. doi: 10.1002/(SICI)1096-9896(199912)189:4<514::AID-PATH463>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gee M. S., Sarkisian C. J., el-Deiry W. S. Identification of a novel AP-2 consensus DNA binding site. Biochem Biophys Res Commun. 1998 Feb 4;243(1):307–316. doi: 10.1006/bbrc.1997.8035. [DOI] [PubMed] [Google Scholar]
- Gilbertson R. J., Perry R. H., Kelly P. J., Pearson A. D., Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997 Aug 1;57(15):3272–3280. [PubMed] [Google Scholar]
- Hennig G., Behrens J., Truss M., Frisch S., Reichmann E., Birchmeier W. Progression of carcinoma cells is associated with alterations in chromatin structure and factor binding at the E-cadherin promoter in vivo. Oncogene. 1995 Aug 3;11(3):475–484. [PubMed] [Google Scholar]
- Hennig G., Löwrick O., Birchmeier W., Behrens J. Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem. 1996 Jan 5;271(1):595–602. doi: 10.1074/jbc.271.1.595. [DOI] [PubMed] [Google Scholar]
- Hietala K. A., Kosma V. M., Syrjänen K. J., Syrjänen S. M., Kellokoski J. K. Correlation of MIB-1 antigen expression with transcription factors Skn-1, Oct-1, AP-2, and HPV type in cervical intraepithelial neoplasia. J Pathol. 1997 Nov;183(3):305–310. doi: 10.1002/(SICI)1096-9896(199711)183:3<305::AID-PATH922>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Jean D., Gershenwald J. E., Huang S., Luca M., Hudson M. J., Tainsky M. A., Bar-Eli M. Loss of AP-2 results in up-regulation of MCAM/MUC18 and an increase in tumor growth and metastasis of human melanoma cells. J Biol Chem. 1998 Jun 26;273(26):16501–16508. doi: 10.1074/jbc.273.26.16501. [DOI] [PubMed] [Google Scholar]
- Karjalainen J. M., Kellokoski J. K., Eskelinen M. J., Alhava E. M., Kosma V. M. Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J Clin Oncol. 1998 Nov;16(11):3584–3591. doi: 10.1200/JCO.1998.16.11.3584. [DOI] [PubMed] [Google Scholar]
- Karjalainen J. M., Kellokoski J. K., Mannermaa A. J., Kujala H. E., Moisio K. I., Mitchell P. J., Eskelinen M. J., Alhava E. M., Kosma V. M. Failure in post-transcriptional processing is a possible inactivation mechanism of AP-2alpha in cutaneous melanoma. Br J Cancer. 2000 Jun;82(12):2015–2021. doi: 10.1054/bjoc.2000.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn M., Scheper G. C., Voorma H. O., Thomas A. A. Regulation of translation initiation factors by signal transduction. Eur J Biochem. 1998 May 1;253(3):531–544. doi: 10.1046/j.1432-1327.1998.2530531.x. [DOI] [PubMed] [Google Scholar]
- Leask A., Byrne C., Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Y., Redmond T. M. Role of the 3'-untranslated region of RPE65 mRNA in the translational regulation of the RPE65 gene: identification of a specific translation inhibitory element. Arch Biochem Biophys. 1998 Sep 1;357(1):37–44. doi: 10.1006/abbi.1998.0817. [DOI] [PubMed] [Google Scholar]
- Nordengren J., Casslén B., Gustavsson B., Einarsdottir M., Willén R. Discordant expression of mRNA and protein for urokinase and tissue plasminogen activators (u-PA, t-PA) in endometrial carcinoma. Int J Cancer. 1998 Apr 17;79(2):195–201. doi: 10.1002/(sici)1097-0215(19980417)79:2<195::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Robertson G. P., Coleman A. B., Lugo T. G. Mechanisms of human melanoma cell growth and tumor suppression by chromosome 6. Cancer Res. 1996 Apr 1;56(7):1635–1641. [PubMed] [Google Scholar]
- Ropponen K. M., Kellokoski J. K., Lipponen P. K., Pietiläinen T., Eskelinen M. J., Alhava E. M., Kosma V. M. p22/WAF1 expression in human colorectal carcinoma: association with p53, transcription factor AP-2 and prognosis. Br J Cancer. 1999 Sep;81(1):133–140. doi: 10.1038/sj.bjc.6690662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sers C., Kirsch K., Rothbächer U., Riethmüller G., Johnson J. P. Genomic organization of the melanoma-associated glycoprotein MUC18: implications for the evolution of the immunoglobulin domains. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8514–8518. doi: 10.1073/pnas.90.18.8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugg S. L., Ezzat S., Rosen I. B., Freeman J. L., Asa S. L. Distinct multiple RET/PTC gene rearrangements in multifocal papillary thyroid neoplasia. J Clin Endocrinol Metab. 1998 Nov;83(11):4116–4122. doi: 10.1210/jcem.83.11.5271. [DOI] [PubMed] [Google Scholar]
- Sun X. F., Carstensen J. M., Zhang H., Stål O., Wingren S., Hatschek T., Nordenskjöld B. Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet. 1992 Dec 5;340(8832):1369–1373. doi: 10.1016/0140-6736(92)92558-w. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Turner B. C., Zhang J., Gumbs A. A., Maher M. G., Kaplan L., Carter D., Glazer P. M., Hurst H. C., Haffty B. G., Williams T. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res. 1998 Dec 1;58(23):5466–5472. [PubMed] [Google Scholar]
- Wang E., Ma W. J., Aghajanian C., Spriggs D. R. Posttranscriptional regulation of protein expression in human epithelial carcinoma cells by adenine-uridine-rich elements in the 3'-untranslated region of tumor necrosis factor-alpha messenger RNA. Cancer Res. 1997 Dec 1;57(23):5426–5433. [PubMed] [Google Scholar]
- Williamson J. A., Bosher J. M., Skinner A., Sheer D., Williams T., Hurst H. C. Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics. 1996 Jul 1;35(1):262–264. doi: 10.1006/geno.1996.0351. [DOI] [PubMed] [Google Scholar]
- Zeng Y. X., Somasundaram K., el-Deiry W. S. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nat Genet. 1997 Jan;15(1):78–82. doi: 10.1038/ng0197-78. [DOI] [PubMed] [Google Scholar]