Abstract
Aim—At present, the diagnosis of muscular dystrophy is made by means of immunohistochemistry on frozen sections. The aim of this study was to develop a sensitive and reproducible immunohistochemical method for use on formalin fixed, paraffin wax embedded sections for the demonstration of dystrophin associated proteins and other muscle associated antigens.
Methods—All the cases studied were from the files of the department of histopathology, Great Ormond Street Hospital for Children NHS Trust. Immunohistochemistry was performed on paraffin wax embedded sections with heat mediated antigen retrieval and overnight incubation with the antibodies at room temperature. Four different pretreatment buffers were tested in the attempt to optimise the immunostaining. Frozen sections were run in parallel for direct comparison.
Results—All the antibodies except δ sarcoglycan gave strong, consistent immunostaining in paraffin wax embedded sections, comparable with the frozen sections. The most consistent results were obtained using citrate/EDTA as the pretreatment buffer.
Conclusion—A reliable and reproducible technique has been established, using a heat mediated citrate/EDTA buffer antigen retrieval method, which works well for most of the antibodies needed to make the diagnosis of muscular dystrophy in formalin fixed, paraffin wax embedded sections. This technique overcomes some of the inherent problems encountered using frozen muscle tissue and it could become a valuable tool for the diagnosis of muscular dystrophy.
Key Words: immunohistochemistry • muscle biopsies • muscular dystrophy • paraffin wax sections
Full Text
The Full Text of this article is available as a PDF (267.8 KB).
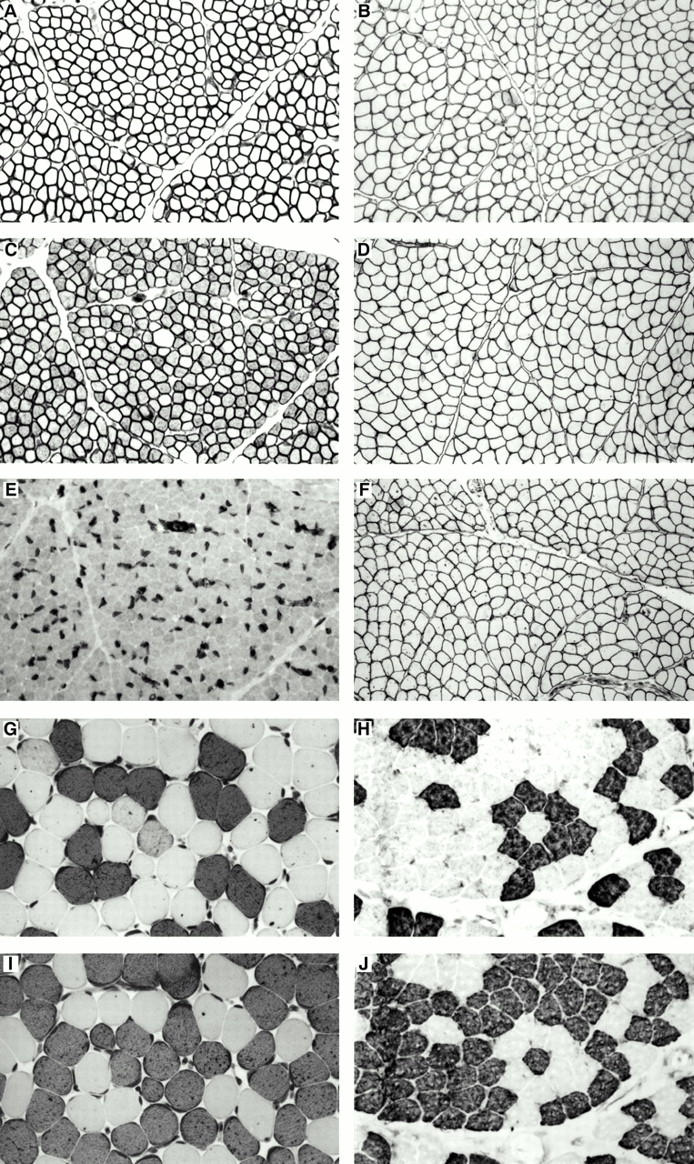
Figure 1 Comparison of paraffin wax embedded sections and frozen sections. Sections were immunostained with antibodies to dystrophin 2 (A, paraffin wax section; B, frozen section), spectrin (C, paraffin wax section; D, frozen section), δ sarcoglycan (E, paraffin wax section; F, frozen section), fast myosin heavy chain (G, paraffin wax section; H, frozen section), and slow myosin heavy chain (I, paraffin wax section; J, frozen section).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER P. E., KIENER F. Eine neue x-chromosomale Muskeldystrophie. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1955;193(4):427–448. doi: 10.1007/BF00343141. [DOI] [PubMed] [Google Scholar]
- Bushby K. M., Beckmann J. S. The limb-girdle muscular dystrophies--proposal for a new nomenclature. Neuromuscul Disord. 1995 Jul;5(4):337–343. doi: 10.1016/0960-8966(95)00005-8. [DOI] [PubMed] [Google Scholar]
- Bushby K. M. Making sense of the limb-girdle muscular dystrophies. Brain. 1999 Aug;122(Pt 8):1403–1420. doi: 10.1093/brain/122.8.1403. [DOI] [PubMed] [Google Scholar]
- Fanin M., Duggan D. J., Mostacciuolo M. L., Martinello F., Freda M. P., Sorarù G., Trevisan C. P., Hoffman E. P., Angelini C. Genetic epidemiology of muscular dystrophies resulting from sarcoglycan gene mutations. J Med Genet. 1997 Dec;34(12):973–977. doi: 10.1136/jmg.34.12.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M. The Wellcome lecture, 1988. Muscular dystrophy: a time of hope. Proc R Soc Lond B Biol Sci. 1989 Jun 22;237(1286):1–9. doi: 10.1098/rspb.1989.0032. [DOI] [PubMed] [Google Scholar]
- Lim L. E., Campbell K. P. The sarcoglycan complex in limb-girdle muscular dystrophy. Curr Opin Neurol. 1998 Oct;11(5):443–452. doi: 10.1097/00019052-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Nicholson L. V., Johnson M. A., Davison K., O'Donnell E., Falkous G., Barron M., Harris J. B. Dystrophin or a "related protein" in Duchenne muscular dystrophy? Acta Neurol Scand. 1992 Jul;86(1):8–14. doi: 10.1111/j.1600-0404.1992.tb08046.x. [DOI] [PubMed] [Google Scholar]
- Ozawa E., Noguchi S., Mizuno Y., Hagiwara Y., Yoshida M. From dystrophinopathy to sarcoglycanopathy: evolution of a concept of muscular dystrophy. Muscle Nerve. 1998 Apr;21(4):421–438. doi: 10.1002/(sici)1097-4598(199804)21:4<421::aid-mus1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Tomé F. M., Evangelista T., Leclerc A., Sunada Y., Manole E., Estournet B., Barois A., Campbell K. P., Fardeau M. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994 Apr;317(4):351–357. [PubMed] [Google Scholar]
- WALTON J. N., NATTRASS F. J. On the classification, natural history and treatment of the myopathies. Brain. 1954;77(2):169–231. doi: 10.1093/brain/77.2.169. [DOI] [PubMed] [Google Scholar]


