Abstract
Aims—To compare the expression of immunohistochemical variables between benign and malignant components of malignant peripheral nerve sheath tumour (MPNST) arising within neurofibroma.
Methods—Eight cases of MPNST arising within a neurofibroma, associated with neurofibromatosis type 1 (NF1), were studied. The areas of MPNST and neurofibroma were compared immunohistochemically with regard to the expression of proliferative activity (MIB-1), growth factors, p53, bcl-2, neural cell adhesion molecule (N-CAM), and CD34.
Results—The expression of transforming growth factor ß1 (TGF-ß1), TGF-ß receptor type II, hepatocyte growth factor α (HGF-α), c-met, p53, and N-CAM was higher in the areas of MPNST than in the neurofibromatous areas in four, five, five, eight, five, and three of the eight cases, respectively. CD34 expression was lower in the areas of MPNST than in the neurofibroma areas in three of the eight cases.
Conclusions—On the basis of these findings, TGF-ß1, HGF-α, and p53 might be involved in the malignant transformation of neurofibroma to MPNST.
Key Words: malignant peripheral nerve sheath tumour • neurofibroma • immunohistochemistry • transforming growth factor ß • hepatocyte growth factor • p53
Full Text
The Full Text of this article is available as a PDF (330.2 KB).
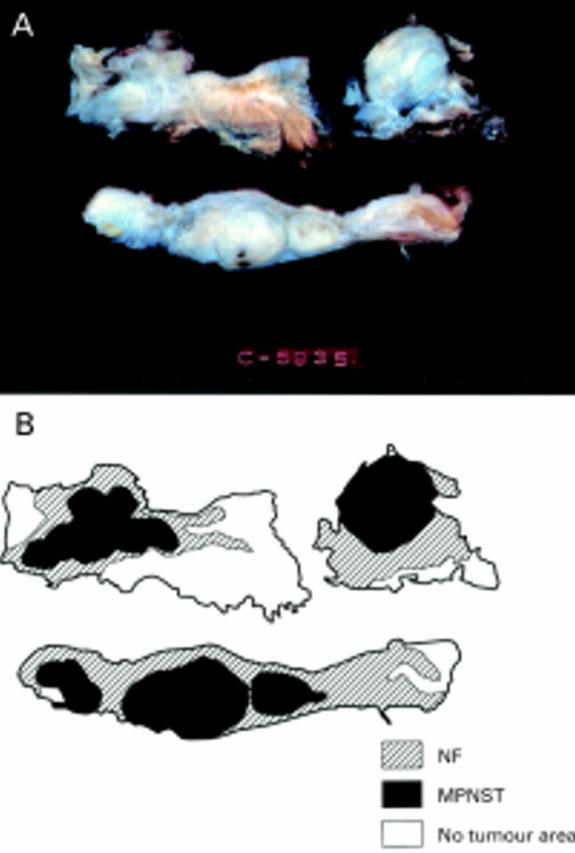
Figure 1 (A) Cut surface of the resected tumour in case 3. Section shows a multinodular solid and white mass. The central firm portion blends with the surrounding subcutaneous fat and muscular tissue. (B) Topographical distribution of histological features of both malignant peripheral nerve sheath tumour and neurofibroma in case 3.
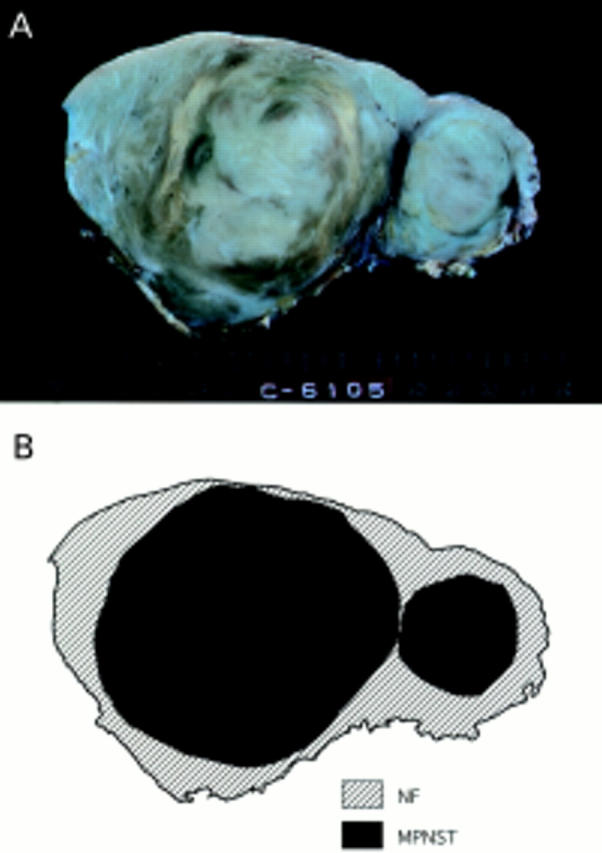
Figure 2 (A) Cut surface of the resected tumour in case 4. The section shows a well circumscribed solid and greyish yellow mass with nodular growth pattern and interlacing fascicles. (B) Topographical distribution of both sarcomatous and neurofibromatous components in case 4.
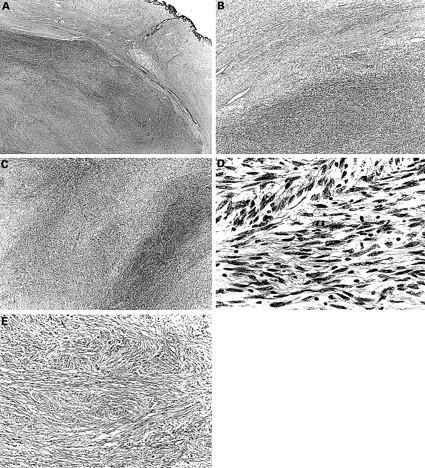
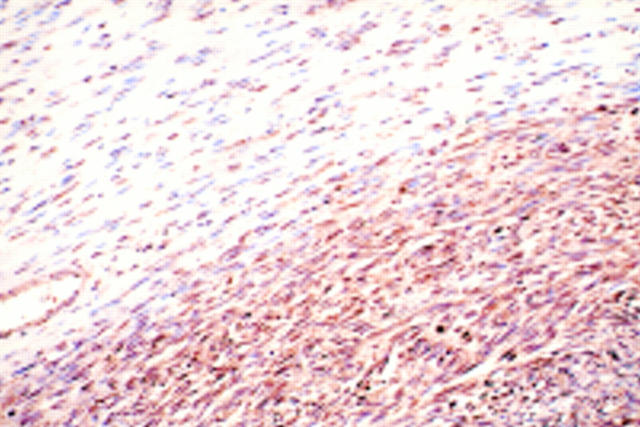
Figure 3 (A–D) Histological picture exhibiting the features of neurofibroma and malignant peripheral nerve sheath tumour (MPNST) (case 4). (A) The MPNST area is surrounded by a neurofibromatous area. Densely cellular fascicles alternate with hypocellular zones in the MPNST area (haematoxylin and eosin stained; original magnification, x12). (B) The tumour is composed of interlacing bundles of elongated cells with wavy nuclei, without atypism in the neurofibroma (top), and with atypism in the MPNST (bottom) (haematoxylin and eosin stained; original magnification, x20). (C) The tumour is composed of wavy spindle cells arranged in fascicles and with wavy nuclei. Densely cellular fascicles alternate with hypocellular zones (haematoxylin and eosin stained; original magnification, x25). (D) The nuclei of some tumour cells are wavy or buckled. Mitotic figures are seen frequently (haematoxylin and eosin stained; original magnification, x570). (E) Histological picture exhibiting the features of neurofibroma (case 4). The tumour is made up of interlacing bundles of elongated cells with wavy nuclei, without atypism, associated with wire-like strands of collagen (haematoxylin and eosin stained; original magnification, x125).
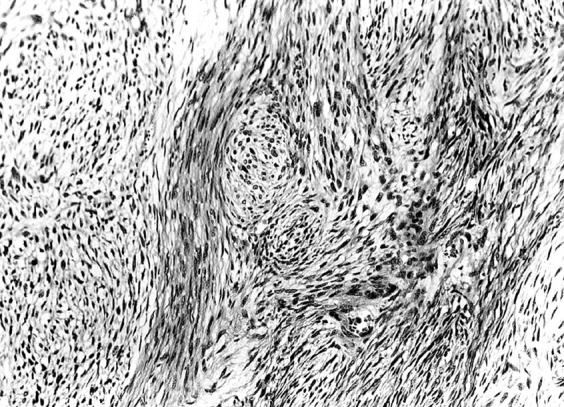
Figure 4 Histological picture exhibiting the features of malignant peripheral nerve sheath tumour (case 5). The tumour is composed of spindle cells arranged in fascicles reminiscent of tactoid differentiation. Nuclei are wavy and twisted (haematoxylin and eosin stained; original magnification, x200).
Figure 5 Immunohistochemical staining patterns of the transforming growth factor ß receptor type II. The degree of staining in the neurofibroma (top) is graded as 1+, whereas that in the MPNST (bottom) is graded as 2+ (case 5) (original magnification, x200).
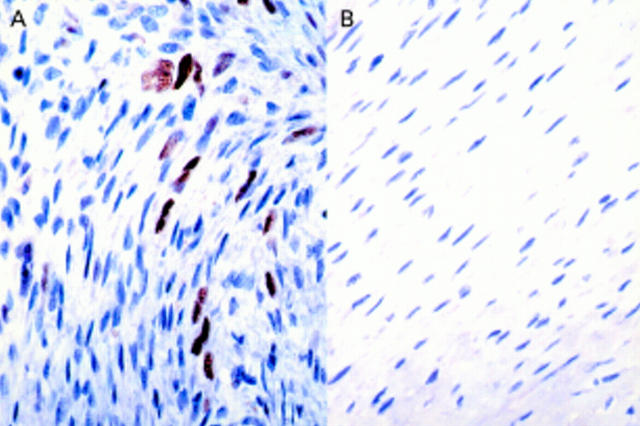
Figure 6 Immunohistochemical staining patterns for p53. Staining in the neurofibroma (B) is negative, whereas that in the MPNST (A) is graded as 1+ (case 8) (original magnification, x400).
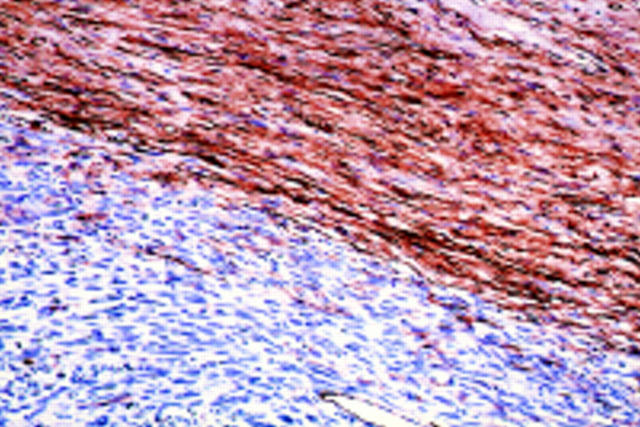
Figure 7 Immunohistochemical staining patterns for CD34. The degree of staining in the neurofibroma (top) is graded as 2+, whereas the MPNST (bottom) is negative (case 5) (original magnification, x200).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daimaru Y., Hashimoto H., Enjoji M. Malignant peripheral nerve-sheath tumors (malignant schwannomas). An immunohistochemical study of 29 cases. Am J Surg Pathol. 1985 Jun;9(6):434–444. doi: 10.1097/00000478-198506000-00005. [DOI] [PubMed] [Google Scholar]
- Davis J. B., Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990 Apr;110(4):1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Papageorge A. G., Fletcher J. A., Diehl S. R., Ratner N., Vass W. C., Lowy D. R. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992 Apr 17;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- Ducatman B. S., Scheithauer B. W., Piepgras D. G., Reiman H. M., Ilstrup D. M. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986 May 15;57(10):2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ebert M., Yokoyama M., Friess H., Büchler M. W., Korc M. Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res. 1994 Nov 15;54(22):5775–5778. [PubMed] [Google Scholar]
- Eccleston P. A., Funa K., Heldin C. H. Expression of platelet-derived growth factor (PDGF) and PDGF alpha- and beta-receptors in the peripheral nervous system: an analysis of sciatic nerve and dorsal root ganglia. Dev Biol. 1993 Feb;155(2):459–470. doi: 10.1006/dbio.1993.1044. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Li L., Schlueter C., Duchrow M., Wohlenberg C., Gerlach C., Stahmer I., Kloth S., Brandt E., Flad H. D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991 Apr;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- Halling K. C., Scheithauer B. W., Halling A. C., Nascimento A. G., Ziesmer S. C., Roche P. C., Wollan P. C. p53 expression in neurofibroma and malignant peripheral nerve sheath tumor. An immunohistochemical study of sporadic and NF1-associated tumors. Am J Clin Pathol. 1996 Sep;106(3):282–288. doi: 10.1093/ajcp/106.3.282. [DOI] [PubMed] [Google Scholar]
- Kindblom L. G., Ahldén M., Meis-Kindblom J. M., Stenman G. Immunohistochemical and molecular analysis of p53, MDM2, proliferating cell nuclear antigen and Ki67 in benign and malignant peripheral nerve sheath tumours. Virchows Arch. 1995;427(1):19–26. doi: 10.1007/BF00203733. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Ehrmann J., Sikorska M., Lach B., Chatten J., Reed J. C. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1, and Bax in tumors of central and peripheral nervous system origin. Am J Pathol. 1997 Mar;150(3):805–814. [PMC free article] [PubMed] [Google Scholar]
- Krasnoselsky A., Massay M. J., DeFrances M. C., Michalopoulos G., Zarnegar R., Ratner N. Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J Neurosci. 1994 Dec;14(12):7284–7290. doi: 10.1523/JNEUROSCI.14-12-07284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legius E., Dierick H., Wu R., Hall B. K., Marynen P., Cassiman J. J., Glover T. W. TP53 mutations are frequent in malignant NF1 tumors. Genes Chromosomes Cancer. 1994 Aug;10(4):250–255. doi: 10.1002/gcc.2870100405. [DOI] [PubMed] [Google Scholar]
- Legius E., Marchuk D. A., Collins F. S., Glover T. W. Somatic deletion of the neurofibromatosis type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat Genet. 1993 Feb;3(2):122–126. doi: 10.1038/ng0293-122. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Saeter G., Danielsen H. E., Stenwig A. E., Høyheim B., O'Connell P., Børresen A. L. Genetic alterations in a malignant schwannoma from a patient with neurofibromatosis (NF1). Pathol Res Pract. 1993 May;189(4):465–474. doi: 10.1016/S0344-0338(11)80339-0. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Slettan A., Saeter G., Brøgger A., Børresen A. L., Nesland J. M. Alterations at chromosome 17 loci in peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 1995 Jan;54(1):65–73. doi: 10.1097/00005072-199501000-00008. [DOI] [PubMed] [Google Scholar]
- McCarron K. F., Goldblum J. R. Plexiform neurofibroma with and without associated malignant peripheral nerve sheath tumor: a clinicopathologic and immunohistochemical analysis of 54 cases. Mod Pathol. 1998 Jul;11(7):612–617. [PubMed] [Google Scholar]
- Menon A. G., Anderson K. M., Riccardi V. M., Chung R. Y., Whaley J. M., Yandell D. W., Farmer G. E., Freiman R. N., Lee J. K., Li F. P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. G., Anderson K. M., Riccardi V. M., Chung R. Y., Whaley J. M., Yandell D. W., Farmer G. E., Freiman R. N., Lee J. K., Li F. P. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Cupo W. Neural cell adhesion molecule distribution in soft tissue tumors. Hum Pathol. 1993 Jan;24(1):62–66. doi: 10.1016/0046-8177(93)90064-n. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lindenmayer A. E., Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994 Jan;7(1):82–90. [PubMed] [Google Scholar]
- Miracco C., Montesco M. C., Santopietro R., Spina D., d'Amore E. S., Tosi P., Ninfo V. Proliferative activity, angiogenesis, and necrosis in peripheral nerve sheath tumors: a quantitative evaluation for prognosis. Mod Pathol. 1996 Dec;9(12):1108–1117. [PubMed] [Google Scholar]
- Naka T., Iwamoto Y., Shinohara N., Ushijima M., Chuman H., Tsuneyoshi M. Expression of c-met proto-oncogene product (c-MET) in benign and malignant bone tumors. Mod Pathol. 1997 Aug;10(8):832–838. [PubMed] [Google Scholar]
- Nakasu S., Nakasu Y., Nioka H., Nakajima M., Handa J. bcl-2 protein expression in tumors of the central nervous system. Acta Neuropathol. 1994;88(6):520–526. doi: 10.1007/BF00296488. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi's sarcoma. Arch Dermatol. 1991 Apr;127(4):523–529. [PubMed] [Google Scholar]
- Rao U. N., Sonmez-Alpan E., Michalopoulos G. K. Hepatocyte growth factor and c-MET in benign and malignant peripheral nerve sheath tumors. Hum Pathol. 1997 Sep;28(9):1066–1070. doi: 10.1016/s0046-8177(97)90060-5. [DOI] [PubMed] [Google Scholar]
- Ratner N., Lieberman M. A., Riccardi V. M., Hong D. M. Mitogen accumulation in von Recklinghausen neurofibromatosis. Ann Neurol. 1990 Mar;27(3):298–303. doi: 10.1002/ana.410270312. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Davis J. B., Stroobant P., Land H. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Stock R., Oda Y., Roessner A. New splicing mutation in exon 5-6 of the p53-tumor suppressor gene in a malignant schwannoma. Hum Mutat. 1997;9(1):91–94. doi: 10.1002/(SICI)1098-1004(1997)9:1<91::AID-HUMU22>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Schubert D. Synergistic interactions between transforming growth factor beta and fibroblast growth factor regulate Schwann cell mitosis. J Neurobiol. 1992 Mar;23(2):143–148. doi: 10.1002/neu.480230205. [DOI] [PubMed] [Google Scholar]
- Serra E., Puig S., Otero D., Gaona A., Kruyer H., Ars E., Estivill X., Lázaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997 Sep;61(3):512–519. doi: 10.1086/515504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela S., Riccardi V. M., Ratner N. Angiogenic and invasive properties of neurofibroma Schwann cells. J Cell Biol. 1990 Aug;111(2):645–653. doi: 10.1083/jcb.111.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse G. R., Kosciolek B. A., Rowley P. T. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosis: loss of heterozygosity for chromosome 17. Genes Chromosomes Cancer. 1989 Sep;1(1):36–41. doi: 10.1002/gcc.2870010107. [DOI] [PubMed] [Google Scholar]
- Skuse G. R., Kosciolek B. A., Rowley P. T. The neurofibroma in von Recklinghausen neurofibromatosis has a unicellular origin. Am J Hum Genet. 1991 Sep;49(3):600–607. [PMC free article] [PubMed] [Google Scholar]
- Taipale J., Saharinen J., Keski-Oja J. Extracellular matrix-associated transforming growth factor-beta: role in cancer cell growth and invasion. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/s0065-230x(08)60740-x. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Swanson P. E., Scheithauer B. W., Manivel J. C. Malignant peripheral nerve sheath tumor. An immunohistochemical study of 62 cases. Am J Clin Pathol. 1987 Apr;87(4):425–433. doi: 10.1093/ajcp/87.4.425. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- von Deimling A., Krone W., Menon A. G. Neurofibromatosis type 1: pathology, clinical features and molecular genetics. Brain Pathol. 1995 Apr;5(2):153–162. doi: 10.1111/j.1750-3639.1995.tb00589.x. [DOI] [PubMed] [Google Scholar]