Abstract
Background/Aims—Fas ligand (FasL) is a mediator of apoptosis via the Fas receptor (Fas/CD95/APO-1). Normal colonic epithelium expresses Fas, and appears to be relatively sensitive to Fas mediated apoptosis. Colonic adenocarcinomas coexpress FasL and Fas without undergoing widespread apoptosis. This study investigates the expression of FasL in colonic carcinogenesis from the earliest stages of the adenoma–carcinoma sequence.
Methods—FasL expression was determined in colonic adenomas (n = 38) of varying degrees of dysplasia and histological type by immunohistochemistry. Adenomas that contained areas of carcinomatous change were included (n = 12 of 38). Normal colonic epithelium (n = 10), hyperplastic polyps (n = 8), and serrated adenomas (n = 3) from patients without colonic adenocarcinomas were used for comparison. Cell death was detected in situ in adenomas using TUNEL (terminal transferase mediated dUTP nick end labelling).
Results—In normal colonic epithelium and hyperplastic polyps, FasL expression was restricted to the luminal surface of the crypts, where Fas–FasL coexpression was coincident with a high frequency of TUNEL positive epithelial cells. All adenomas (n = 38) had an altered distribution of positive FasL staining; FasL expression was found in most cells (> 70% of neoplastic cells). Expression of Fas was also detected throughout the adenomas, but coexpression of FasL and Fas was not associated with TUNEL positivity in most cells.
Conclusions—FasL upregulation occurs early in the adenoma–carcinoma sequence of colon carcinogenesis, and is evident at the level of mild dysplasia. The lack of pronounced apoptosis in areas of adenomas coexpressing Fas and FasL suggests that colonocytes acquire resistance to Fas mediated apoptosis early in the transformation process.
Key Words: Fas (CD95/APO-1) • Fas ligand • colon • adenoma
Full Text
The Full Text of this article is available as a PDF (1.1 MB).
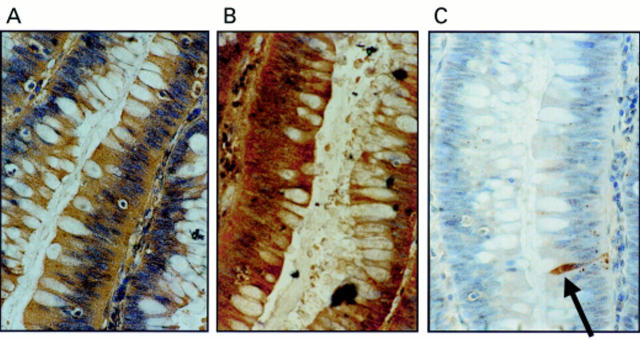
Figure 1 Coexpression of Fas ligand (FasL) and Fas in colonic adenomas is not associated with Fas mediated apoptosis. (A) Immunoperoxidase staining using a FasL specific rabbit polyclonal antibody was performed on paraffin wax embedded colonic adenoma sections. Slides were counterstained with haematoxylin. FasL positive immunohistochemical staining (brown) is shown in a representative mildly dysplastic villous adenoma. As a control for specificity of antibody detection, the FasL immunising peptide was included during primary antibody incubation. Competitive displacement of staining by the immunising peptide confirmed FasL specificity (not shown). (B) Another section sequential to that shown in (A) was used to detect Fas, using immunoperoxidase staining with a Fas specific rabbit polyclonal antibody. Fas positive immunohistochemical staining (brown) was present in the adenoma cells. (C) Cell death detection in situ by terminal transferase mediated dUTP nick end labelling (TUNEL). Sections sequential to those shown in (A) and (B) were used to detect cell death by enzymic labelling of DNA strand breaks using TUNEL. Only those cells with positive TUNEL staining (brown) and exhibiting apoptotic morphology (arrow) were considered to be apoptotic. Only occasional TUNEL positive cells were identified despite widespread Fas–FasL coexpression. Control sections (not shown) where the terminal transferase was omitted were negative. These results are representative of 38 colonic adenomas.
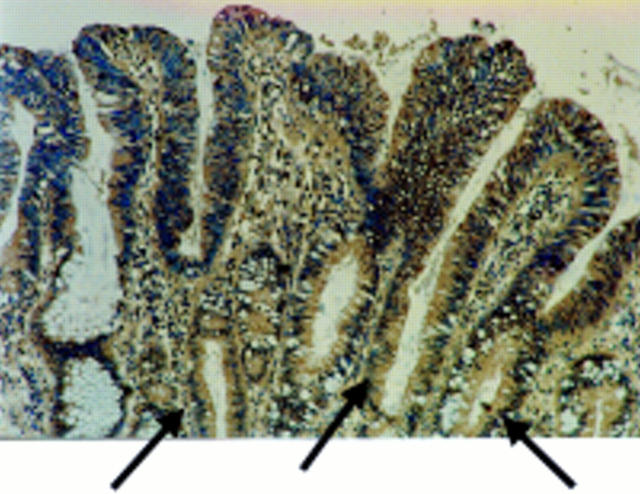
Figure 2 Fas ligand (FasL) is expressed in areas of peritumoral epithelium in colonic adenocarcinomas. Immunoperoxidase staining using a FasL specific rabbit polyclonal antibody was performed on paraffin wax embedded colonic adenocarcinoma sections. Slides were counterstained with haematoxylin. FasL positive immunohistochemical staining (brown) is shown in a representative area of peritumoral epithelium. As a control for specificity of antibody detection, the FasL immunising peptide was included during primary antibody incubation. Competitive displacement of staining by the immunising peptide confirmed FasL specificity (not shown). FasL expression by the neoplastic cells extends into the crypts of the dysplastic glands (arrows). This result is representative of peritumoral epithelium from 30 of 32 colonic carcinomas that showed increased FasL expression.
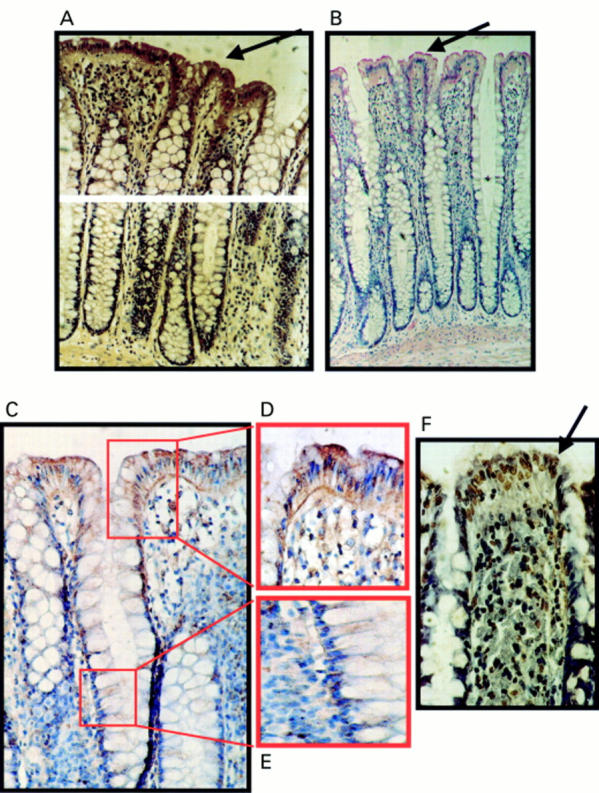
Figure 3 Expression of Fas ligand (FasL) at the luminal surface of normal colonic epithelium coincides with increased frequency of terminal transferase mediated dUTP nick end labelling (TUNEL) positive epithelial cells. (A) Immunoperoxidase staining using a FasL specific rabbit polyclonal antibody was performed on paraffin wax embedded normal colonic sections. Slides were counterstained with haematoxylin. FasL positive immunohistochemical staining (brown) is shown in a representative normal colonic specimen. FasL expression was detected in luminal colonic epithelium (arrow). As a control for specificity of antibody detection, the FasL immunising peptide was included during primary antibody incubation. Competitive displacement of staining by the immunising peptide confirmed FasL specificity (not shown). (B) FasL immunophosphatase (alkaline phosphatase conjugated anti-alkaline phosphatase; APAAP) staining (red) was performed using a FasL specific monoclonal antibody. Slides were counterstained with haematoxylin. Results from staining with the FasL specific monoclonal antibody agreed with those obtained using the polyclonal antibody—FasL positive epithelial cells (red; arrow) were present at the luminal surface. (C) Immunoperoxidase staining with the same FasL specific monoclonal antibody as used in (B) gave identical results. Thus, staining was independent of secondary detection systems because similar results were obtained using either APAAP or immunoperoxidase assays. (D) FasL positive luminal epithelium (brown) from (C) illustrated at higher magnification. (E) Negative crypt epithelium from (C) illustrated at higher magnification. (F) Cell death detection in situ by TUNEL. Cell death was detected by enzymic labelling of DNA strand breaks using TUNEL in normal colonic sections sequential to those shown in (A). Only those cells with positive TUNEL staining (brown) and exhibiting apoptotic morphology (arrow) were considered to be apoptotic. Control sections (not shown) where the terminal transferase was omitted were negative. An increased frequency of TUNEL positive epithelial cells was detected at the luminal surface, where cell death was coincident with FasL expression. These results are representative of 20 normal colonic specimens.
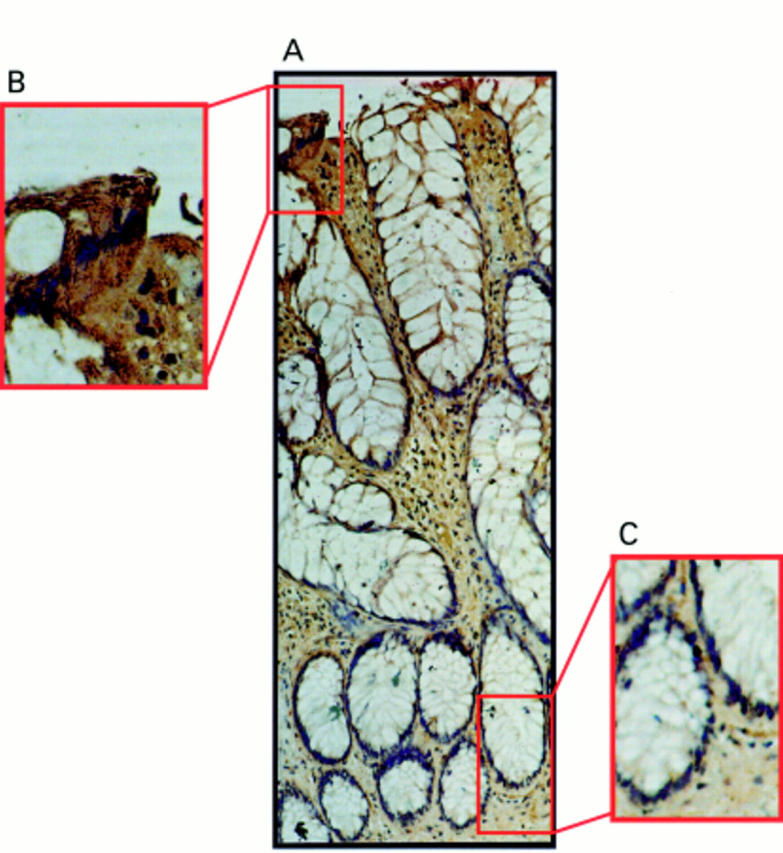
Figure 4 Fas ligand (FasL) expression occurs only at the luminal surface of hyperplastic polyps. (A) Immunoperoxidase staining was performed in paraffin wax embedded hyperplastic polyp sections using a FasL specific polyclonal antibody. Slides were counterstained with haematoxylin. FasL positive immunohistochemical staining (brown) is shown in a representative hyperplastic polyp. The pattern of FasL immunoreactivity was similar to that seen in normal epithelium, with FasL expression occurring in epithelial cells at the luminal surface, but being absent from the crypt base. (B) Detection of FasL positivity in luminal epithelium illustrated at higher magnification. (C) FasL negative crypt epithelium illustrated at higher magnification. These results are representative of 10 hyperplastic polyps.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Suematsu S., Kondo T., Ogasawara J., Tanaka T., Yoshida N., Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995 Nov;11(3):294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Bennett M. W., O'Connell J., O'Sullivan G. C., Roche D., Brady C., Collins J. K., Shanahan F. Fas ligand and Fas receptor are coexpressed in normal human esophageal epithelium: a potential mechanism of apoptotic epithelial turnover. Dis Esophagus. 1999;12(2):90–98. doi: 10.1046/j.1442-2050.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- Berthou C., Michel L., Soulié A., Jean-Louis F., Flageul B., Dubertret L., Sigaux F., Zhang Y., Sasportes M. Acquisition of granzyme B and Fas ligand proteins by human keratinocytes contributes to epidermal cell defense. J Immunol. 1997 Dec 1;159(11):5293–5300. [PubMed] [Google Scholar]
- Charriaut-Marlangue C., Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995 Dec 29;7(1):61–64. [PubMed] [Google Scholar]
- Cho K. R., Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992 Sep 15;70(6 Suppl):1727–1731. doi: 10.1002/1097-0142(19920915)70:4+<1727::aid-cncr2820701613>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- French L. E., Hahne M., Viard I., Radlgruber G., Zanone R., Becker K., Müller C., Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996 Apr;133(2):335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. S., Brunner T., Fletcher S. M., Green D. R., Ferguson T. A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995 Nov 17;270(5239):1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Ferguson T. A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997 May;18(5):240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- Griffith T. S., Yu X., Herndon J. M., Green D. R., Ferguson T. A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996 Jul;5(1):7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Coates P. J., Ansari B., Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994 Dec;107(Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- Lee J., Richburg J. H., Younkin S. C., Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997 May;138(5):2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Longacre T. A., Fenoglio-Preiser C. M. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990 Jun;14(6):524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- Lynch D. H., Ramsdell F., Alderson M. R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995 Dec;16(12):569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Mizuno M., Shimizu M., Manabe T., Iida M. Clinicopathological features of serrated adenoma of the colorectum: comparison with traditional adenoma. J Clin Pathol. 1999 Jul;52(7):513–516. doi: 10.1136/jcp.52.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morson B. C. The evolution of colorectal carcinoma. Clin Radiol. 1984 Nov;35(6):425–431. doi: 10.1016/s0009-9260(84)80033-1. [DOI] [PubMed] [Google Scholar]
- Möller P., Koretz K., Leithäuser F., Brüderlein S., Henne C., Quentmeier A., Krammer P. H. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994 May 1;57(3):371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- Niehans G. A., Brunner T., Frizelle S. P., Liston J. C., Salerno C. T., Knapp D. J., Green D. R., Kratzke R. A. Human lung carcinomas express Fas ligand. Cancer Res. 1997 Mar 15;57(6):1007–1012. [PubMed] [Google Scholar]
- O'Connell J., Bennett M. W., Nally K., Houston A., O'Sullivan G. C., Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000 Jun;910:178–195. doi: 10.1111/j.1749-6632.2000.tb06708.x. [DOI] [PubMed] [Google Scholar]
- O'Connell J., Bennett M. W., O'Sullivan G. C., Collins J. K., Shanahan F. Fas counter-attack--the best form of tumor defense? Nat Med. 1999 Mar;5(3):267–268. doi: 10.1038/6477. [DOI] [PubMed] [Google Scholar]
- O'Connell J., Bennett M. W., O'Sullivan G. C., Roche D., Kelly J., Collins J. K., Shanahan F. Fas ligand expression in primary colon adenocarcinomas: evidence that the Fas counterattack is a prevalent mechanism of immune evasion in human colon cancer. J Pathol. 1998 Nov;186(3):240–246. doi: 10.1002/(SICI)1096-9896(199811)186:3<240::AID-PATH173>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G. C., Collins J. K., Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996 Sep 1;184(3):1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Schaub L. B., Zhang W., Cusack J. C., Angelo L. S., Santee S. M., Fujiwara T., Roth J. A., Deisseroth A. B., Zhang W. W., Kruzel E. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995 Jun;15(6):3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runic R., Lockwood C. J., Ma Y., Dipasquale B., Guller S. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab. 1996 Aug;81(8):3119–3122. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- Saas P., Walker P. R., Hahne M., Quiquerez A. L., Schnuriger V., Perrin G., French L., Van Meir E. G., de Tribolet N., Tschopp J. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997 Mar 15;99(6):1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg P. R., Saporta S., Borlongan C. V., Othberg A. I., Allen R. C., Cameron D. F. The testis-derived cultured Sertoli cell as a natural Fas-L secreting cell for immunosuppressive cellular therapy. Cell Transplant. 1997 Mar-Apr;6(2):191–193. doi: 10.1177/096368979700600213. [DOI] [PubMed] [Google Scholar]
- Shiraki K., Tsuji N., Shioda T., Isselbacher K. J., Takahashi H. Expression of Fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci U S A. 1997 Jun 10;94(12):6420–6425. doi: 10.1073/pnas.94.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand S., Hofmann W. J., Hug H., Müller M., Otto G., Strand D., Mariani S. M., Stremmel W., Krammer P. H., Galle P. R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells--a mechanism of immune evasion? Nat Med. 1996 Dec;2(12):1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- Sträter J., Wellisch I., Riedl S., Walczak H., Koretz K., Tandara A., Krammer P. H., Möller P. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997 Jul;113(1):160–167. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Enari M., Eguchi Y., Matsuzawa A., Nagata S., Tsujimoto Y., Iguchi T. Involvement of Fas in regression of vaginal epithelia after ovariectomy and during an estrous cycle. EMBO J. 1996 Jan 15;15(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- Watson A. J. Review article: manipulation of cell death--the development of novel strategies for the treatment of gastrointestinal disease. Aliment Pharmacol Ther. 1995 Jun;9(3):215–226. doi: 10.1111/j.1365-2036.1995.tb00376.x. [DOI] [PubMed] [Google Scholar]
- Winawer S. J., Zauber A. G., Ho M. N., O'Brien M. J., Gottlieb L. S., Sternberg S. S., Waye J. D., Schapiro M., Bond J. H., Panish J. F. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- Winawer S. J., Zauber A. G., O'Brien M. J., Gottlieb L. S., Sternberg S. S., Stewart E. T., Bond J. H., Schapiro M., Panish J. F., Waye J. D. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer. 1992 Sep 1;70(5 Suppl):1236–1245. doi: 10.1002/1097-0142(19920901)70:3+<1236::aid-cncr2820701508>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Bellamy C. O., Bubb V. J., Clarke A. R., Corbet S., Curtis L., Harrison D. J., Hooper M. L., Toft N., Webb S. Apoptosis and carcinogenesis. Br J Cancer. 1999 Jul;80 (Suppl 1):34–37. [PubMed] [Google Scholar]