Abstract
Cancer–testis antigen NY-ESO-1 is one of the most immunogenic tumor antigens defined to date. Spontaneous humoral and CD8+ T-cell responses to NY-ESO-1 are detected in 40–50% of patients with advanced NY-ESO-1-expressing tumors. A clinical trial was initiated to study the immunological effects of intradermal vaccination with 3 HLA-A2-binding NY-ESO-1 peptides in 12 patients with metastatic NY-ESO-1-expressing cancers. Seven patients were NY-ESO-1 serum antibody negative, and five patients were NY-ESO-1 serum antibody positive at the outset of the study. Primary peptide-specific CD8+ T-cell reactions and delayed-type hypersensitivity responses were generated in four of seven NY-ESO-1 antibody-negative patients. Induction of a specific CD8+ T-cell response to NY-ESO-1 in immunized antibody-negative patients was associated with disease stabilization and objective regression of single metastases. NY-ESO-1 antibody-positive patients did not develop significant changes in baseline NY-ESO-1-specific T-cell reactivity. However, stabilization of disease and regression of individual metastases were observed in three of five immunized patients. These results demonstrate that primary NY-ESO-1-specific CD8+ T-cell responses can be induced by intradermal immunization with NY-ESO-1 peptides, and that immunization with NY-ESO-1 may have the potential to alter the natural course of NY-ESO-1-expressing tumors.
Analysis of spontaneous immune responses against cancer in humans has led to the identification of a large number of tumor antigens (1). The majority of these antigens can be classified into one of the following categories according to their expression pattern, function, or origin: cancer–testis (CT) antigens, e.g., MAGE (2, 3) and NY-ESO-1 (4), which are aberrantly expressed in tumor cells but that, with the exception of germ cells, are silent in normal cells; differentiation antigens of the melanocyte lineage, e.g., Melan A/MART-1 (5, 6), tyrosinase (7), and gp100 (8, 9); mutational antigens, e.g., MUM-1 (10), p53 (11, 12), and CDK4 (13); overexpressed “self” antigens, e.g., HER2/neu (14) and p53 (12); and viral antigens, e.g., HPV (15) and EBV (16). Spontaneous immune responses elicited by these antigens are either predominantly cellular, e.g., tyrosinase (17, 18) and Melan A/MART-1 (9, 19), or are associated with a strong humoral immune component, e.g., NY-ESO-1 (20) and p53 (12).
NY-ESO-1 is a highly immunogenic CT antigen, inducing simultaneous cellular and humoral immune responses in a high percentage of patients with advanced NY-ESO-1-expressing tumors (20, 21). Detectable NY-ESO-1 serum antibody depends on the presence of NY-ESO-1-expressing tumor, and antibody titers correlate with the clinical development of disease (20, 22). NY-ESO-1-specific CD8+ T-cell responses were detected in more than 90% of NY-ESO-1 antibody-positive patients, whereas NY-ESO-1 antibody-negative patients showed no detectable NY-ESO-1-specific T-cell reactivity (23).
The present study was initiated to evaluate the effects of active immunization with NY-ESO-1 peptides in NY-ESO-1 antibody-negative and -positive patients. Three naturally processed NY-ESO-1 peptides presented by HLA-A2 were used for intradermal immunization, first alone and then in combination with granulocyte–macrophage colony-stimulating factor (GM-CSF) as a systemic adjuvant. The following parameters were monitored in this trial: (i) peptide-specific CD8+ T-cell responses; (ii) delayed-type hypersensitivity (DTH) reactivity; (iii) NY-ESO-1-specific antibody responses; and (iv) disease status.
Methods
Immunization Protocol.
Twelve HLA-A2+ patients with progressing NY-ESO-1-expressing metastatic tumors of different types and meeting predefined entry criteria were selected for immunization in the LUD97-008 protocol sponsored by the Ludwig Institute for Cancer Research. Immunizations were performed with three HLA-A2-binding NY-ESO-1 peptides derived from NY-ESO-1 and initially identified by the T-cell line NW38-IVS-1 (21). The NY-ESO-1 peptide sequences were: p157–167 (SLLMWITQCFL), p157–165 (SLLMWITQC), and p155–163 (QLSLLMWIT). The HLA-A2-presented influenza matrix peptide p58–66 (GILGFVFTL) was used as a positive control for immune responses in vitro and in vivo. Peptides (>90% purity) were manufactured according to good manufactorial practice guidelines (Multiple Peptide Systems, San Diego) and solubilized in 100% DMSO. Intradermal injection of the 100% DMSO/peptide solution caused an immediate nonspecific skin reaction. For this reason, the peptide solution was diluted with PBS to a final concentration of 33% DMSO, and this concentration of DMSO caused no local toxic skin reaction. For immunization, the total dose of 100 μg of each peptide solubilized in a final volume of 0.9 ml 33% DMSO was divided in 3 portions of 0.3 ml each and was injected intradermally at separate sites once weekly for 4 weeks. Patients with no evidence of disease progression on day 50 received further immunizations by using the peptides at the same dose and schedule, combined with GM-CSF administered s.c. at 75 μg/day from day −3 to day +2 in relation to each peptide injection.
NY-ESO-1 Antibody.
Serum antibody against the recombinant NY-ESO-1 protein was measured by standard Western blot analysis and ELISA by using NY-ESO-1 recombinant protein purified from Escherichia coli as described (20).
Peptide Presensitization.
Purified CD8+ T lymphocytes were presensitized with peptide-pulsed irradiated autologous peripheral blood lymphocytes depleted of CD4+ and CD8+ T cells as described (23). Presensitized CD8+ T cells were used as effectors on day 6 for enzyme-linked immunospot (ELISPOT) analysis or restimulated on day 7 for the assessment of cytotoxicity against peptide-pulsed T2 cells (day 12) or melanoma cells (day 13) in chromium-51 release assays (23).
ELISPOT Assay.
The frequency of NY-ESO-1-specific CD8+ T cells in the peripheral blood of patients was assessed by ELISPOT as previously described (23). The number of blue spots per well was determined and the results recorded as the average of duplicate wells.
Cytotoxicity Assay.
Cytotoxicity against peptide-pulsed T2 cells and tumor cells was determined in standard chromium release assays as described (21). Unlabeled K562 (40:1) were added to the target cells to block nonspecific cytotoxicity. Tumor cell lines used as targets in cytotoxicity assays were MZ-MEL-19, NW-MEL-38, SK-MEL-37, and NW-MEL-145.
Disease Assessment.
The assessment of individual tumor lesions was performed according to World Health Organization criteria: complete remission, a complete regression of the tumor mass; partial remission, a >50% regression of the tumor mass; minor remission, a 25–50% regression of the tumor mass; stable disease, a +/−25% regression or progression of the tumor mass; and progressive disease, a >25% progression of the tumor mass or the occurrence of new lesions.
Results
The 12 HLA-A2+ patients included in this series had progressive NY-ESO-1-expressing tumors. At the time of study entry, seven of the patients had no detectable NY-ESO-1 antibody or CD8+ T-cell reactivity to HLA-A2-restricted NY-ESO-1 peptides, and five of the patients had NY-ESO-1-antibody and NY-ESO-1-specific CD8+ T-cell reactivity. Fig. 1 provides a schematic summary of immunologic and clinical parameters monitored during NY-ESO-1 peptide vaccination.
Figure 1.
Immunological and clinical effects of vaccination in NY-ESO-1 seronegative (A) and seropositive (B) patients. ELISPOT assays: number of IFN-γ-releasing CD8+ T lymphocytes per 2.5 × 104 CD8+ T cells after presensitization with two NY-ESO-1 peptides (11 mer, SLLMWITQCFL; p157–167; 9 mer, SLLMWITQC; p157–165). No measurable CD8+ T-cell responses were found against the third NY-ESO-1 peptide used for immunization (QLSLLMWIT; p155–163) or against an irrelevant peptide (GILTVILGV; p29–37) used as a negative control in the assays (data not shown). ne, not evaluable because of high background reactivity. Cytotoxicity against peptide-pulsed T2 cells: +, >20% specific lysis above background. DTH reactions classified according to the area of redness and induration: −, no reaction; −/+, weak or questionable reaction; white circle, positive reaction >8 mm; red circle, inflammatory reaction >2 cm. ns, immediate nonspecific skin reaction after injection of 100% DMSO/peptide solution. The development of single metastases is documented according to the World Health Organization classification of response as described. Abbreviations: nd, not done; LU, lung; CNS, brain; Pl, pleura; SC, s.c.; LN, lymph node; LI, liver; P, peritoneum. *, The designation “progressive disease” (PD) was given because of an enlargement of the lesion. However, this enlargement was attributable to increasing necrosis of the s.c. mass. Additional cycles of vaccination were carried out in patients NW415 and NW886 (total number of cycles, five) and in patient NW924 (total number of cycles, four).
Induction of Peptide-Specific CD8+ T-Cell Reactivity: ELISPOT Assays.
Antibody-negative patients.
Fig. 1A provides a summary of the response patterns of NY-ESO-1 antibody-negative patients to NY-ESO-1 peptide vaccination. Strong ELISPOT reactivity against the NY-ESO-1 11-mer peptide (p157–167) was observed in four of seven vaccinated patients. Reactivity against the NY-ESO-1 9-mer peptide (p157–165) appeared later and at a lower level. No ELISPOT reactivity was detected against the second NY-ESO-1 9-mer peptide (p155–163) in any of the vaccinated patients. In patient NW415, there was a rapid induction of strong 11-mer reactivity, a slower induction of 9-mer reactivity, and persistence of both 11- and 9-mer reactivity throughout the course of vaccination (five cycles). The same general pattern was observed in patients NW745, NW836, and NW886, but the response to both the 11- and 9-mer was delayed, occurring at a later interval after vaccination than in patient NW415. To confirm that the rapid induction of CD8+ T-cell responses in patient NW415 was a consequence of peptide vaccination, a sample of lymphocytes obtained 3 months before vaccination was analyzed. No NY-ESO-1-specific CD8+ T-cell reactivity was detectable. The HLA-A2-restricted influenza matrix peptide and an irrelevant peptide were used as specificity controls in ELISPOT assays. A strong influenza response (>500 spots/2.5 × 104 CD8+ T cells) was observed at all time points tested in all patients, whereas no reactivity against the irrelevant peptide was observed. ELISPOT assays with CD8+ T cells from patient NW989 could not be evaluated because of high persistent background reactivity of unknown origin.
Antibody-positive patients.
Four of five patients in the series with NY-ESO-1 antibody showed strong CD8+ T-cell reactivity to NY-ESO-1 before vaccination (Fig. 1b). In these patients, spontaneous CD8+ T-cell reactivity against the NY-ESO-1 9-mer peptide (p157–165) was significantly greater than against the NY-ESO-1 11-mer peptide (p157–167). This is the opposite reactivity pattern observed in antibody-negative patients vaccinated with NY-ESO-1 peptides, where the 11-mer reactivity is greater than the 9-mer reactivity. Peptide vaccination of antibody-positive patients induced a clear 11-mer reactivity in 1 patient with initially no 11-mer reactivity at study onset (NW924) but did not change the 9-mer reactivity in any patient.
Peptide-Specific CD8+ T-Cell Responses: Cytotoxicity Assays.
Antibody-negative patients.
Fig. 1A summarizes the results of cytotoxicity tests with CD8+ T cells from patients vaccinated with NY-ESO-1 peptides. A de novo induction of NY-ESO-1-specific cytotoxicity was clearly observed in patient NW415, first against the 11-mer-pulsed target cells and then against the 9-mer-pulsed target cells. CD8+ T cells with reactivity against the 11 mer were also induced in patients NW745 and NW836 but at a later time than in patient NW415.
Antibody-positive patients.
With the exception of patients NW889 and NW924, the other 3 patients had strong specific CD8+ T-cell cytotoxicity against 9- and 11-mer peptide-pulsed cells before vaccination. Vaccination with NY-ESO-1 peptides did not alter this pattern of CD8+ T-cell cytotoxicity in antibody-positive patients (Fig. 1B).
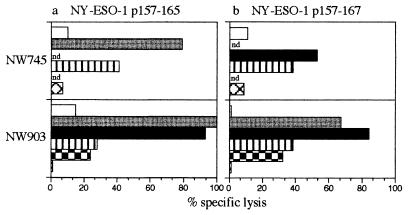
Fig. 2 shows cytotoxicity tests with CD8+ T cells from two vaccinated patients: NW745 (initially antibody negative) and NW903 (initially antibody positive). T cells used for these assays were collected on days 127 (NW745) and 78 (NW903) after the first vaccination. T2 cells pulsed with NY-ESO-1 11-mer (p157–167) or 9-mer (p157–165) peptides or NY-ESO-1-expressing or -nonexpressing melanoma cells were used as targets. CD8+ T cells from both antibody-negative and antibody-positive patients lysed 11- and 9-mer peptide-pulsed cells and NY-ESO-1+ melanoma cells (NW-MEL-38, SK-MEL-37). NY-ESO-1-negative melanoma cells (NW-MEL-145) were not lysed.
Figure 2.
NY-ESO-1-specific cytotoxicity of CD8+ T lymphocytes of patient NW745 (lymphocytes obtained on day 127 of immunization) and patient NW903 (lymphocytes obtained on day 78 of immunization) after presensitization with the NY-ESO-1 nonamer (p157–165) (a) and the NY-ESO-1 11-mer (p157–167) (b) peptides. Target cells were T2 cells alone (white bars); T2 cells pulsed with the NY-ESO-1 9 mer (p157–165; gray bars); T2 cells pulsed with the NY-ESO-1 11 mer (p157–167; black bars); NY-ESO-1+, HLA-A2+ melanoma cell lines NW-MEL-38 (striped bars); SK-MEL-37 (checkered bars); and NY-ESO-1−, HLA-A2+ melanoma cell line NW-MEL-145 (crossed bars). The chromium-51 release assay is described in Materials and Methods. nd, not done.
DTH Reactions.
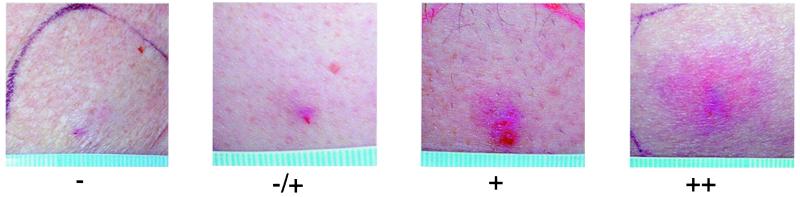
DTH reactions were assessed in all patients 48 h after each immunization. The area of redness and palpable induration and the inflammatory characteristics of the reaction were evaluated. Nonspecific skin reactions associated with 100% DMSO were characterized by the lack of induration and inflammation. The scoring system for DTH reactions is presented in Fig. 3.
Figure 3.
Classification of DTH reactions induced by the NY-ESO-1 11-mer peptide (p165–167). Reaction patterns: −, no detectable redness or induration; −/+, weak or questionable skin reaction with redness <8 mm in diameter and without palpable induration; +, redness and induration >8 mm in diameter; ++, inflammatory reaction >2 cm in diameter.
In antibody-negative patients, the first injection of NY-ESO-1 peptides elicited little or no DTH reactivity. In contrast, a strong DTH reaction was induced by the influenza matrix peptide in these patients. The continued vaccination with NY-ESO-1 peptides was associated with a progressive increase in DTH reactivity in five of seven patients, with strongest DTH reactions elicited by the 11-mer (p157–167), less reactivity to the 9-mer (p157–165), and no reactivity to the second NY-ESO-1 9-mer peptide (p155–163). DTH reactivity generally began to appear after two to four peptide vaccinations and was further amplified by the administration of GM-CSF. In contrast to the antibody-negative patients, strong inflammatory reactions were elicited in two of five antibody-positive patients by the initial NY-ESO-1 peptide injection. Similar to the observation in the antibody-negative patients, the intensity of DTH reactions was greatly enhanced by GM-CSF administration. In patients NW745, NW836, and NW731, it was not possible to evaluate DTH reactions to the first two peptide vaccines because of the immediate nonspecific local skin irritation caused by the undiluted DMSO/peptide solution used during the initial phase of the study. However, use of 33% DMSO to solubilize the peptides eliminated this problem, and subsequent injections elicited strong inflammatory DTH reactions (NW745, fourth injection; NW836, fourth injection; NW731, third injection).
NY-ESO-1-Specific Humoral Immune Response Secondary to the Induction of NY-ESO-1-Specific CD8+ T-Cell Reactivity.
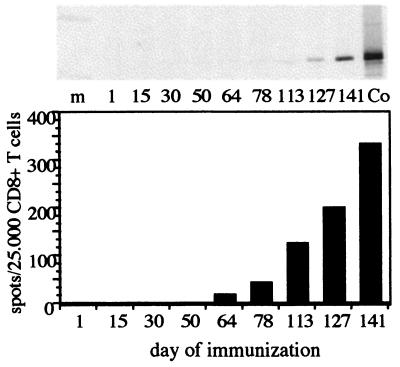
All antibody-negative patients were tested for NY-ESO-1 antibody throughout the course of NY-ESO-1 peptide vaccination, and one patient was found to undergo a NY-ESO-1 seroconversion. Melanoma patient NW745 developed a gradually increasing antibody response against NY-ESO-1, first detected in Western blot and ELISA on day 113 of vaccination with NY-ESO-1 peptides (Fig. 4 Upper). The humoral immune response was preceded by the development of a NY-ESO-1-specific CD8+ T-cell response first detected on day 64 (Fig. 4 Lower).
Figure 4.
(Upper) Development of NY-ESO-1 serum antibody during immunization of patient NW745 with NY-ESO-1 peptides as demonstrated by Western blot. NY-ESO-1 antibody was first detected on day 113 and showed an increasing titer with continued immunization. Serum dilution was 1:250. Co, blot with 0,25 μg of NY-ESO-1 recombinant “short” protein (14 kDa) and serum from a melanoma patient with NY-ESO-1 antibody. (Lower) Development of NY-ESO-1 CD8+ T cells during immunization of patient NW745 with NY-ESO-1 peptides as demonstrated by ELISPOT analysis. CD8+ T-cell reactivity was first detected on day 64 and increased in frequency with continued immunization. The CD8+ T-cell response preceded the development of NY-ESO-1 antibody in patient NW745.
Disease Status.
All patients had documented disease progression during the 4 weeks before study entry. The status of individual metastatic lesions was assessed before and after each vaccine cycle (Fig. 1 A and B). Five of seven vaccinated patients who were initially NY-ESO-1 antibody negative developed stabilization or regression of individual metastases according to World Health Organization (WHO) criteria. Melanoma patient NW415 had clinical stabilization of lymph node metastases for 8 months during 5 cycles of NY-ESO-1 peptide vaccinations. A new lymph node metastasis was detected on day 258, and the patient was accordingly removed from the study. Melanoma patient NW745 showed a minor regression of one lesion and no measurable change in three other lesions during three cycles of NY-ESO-1 vaccination. In addition, extensive necrosis was observed in one lymph node metastasis and in a s.c. lesion during the second and third vaccine cycles. Biopsies of the lymph node metastasis were obtained before vaccination and after 20 weeks of vaccination. Increased intratumoral infiltrates of CD4+ and CD8+ T lymphocytes were found in the postvaccine biopsy at a time when NY-ESO-1-specific CD8+ T cells were detectable in the peripheral blood. The s.c. lesion was assessed “progressive disease” according to WHO classification on day 141. However, enlargement of the lesion was attributable to increasing central necrosis. At the patient's request, treatment was discontinued on day 150 of the study. Melanoma patient NW836 had stabilization of two s.c. metastases during two cycles of vaccination. After day 78, a new s.c. lesion was diagnosed, and vaccination was discontinued. Melanoma patient NW46 had a partial regression of an individual lymph node and a complete regression of a s.c. metastasis during two cycles of vaccination. However, brain metastases developed on day 112, and the vaccination was stopped and radiation of the central nervous system was initiated. Melanoma patient NW789 had progressive disease after one cycle of immunization and was removed from the study. Patient NW989 with ovarian carcinoma had stable peritoneal disease with decreasing malignant ascites during three cycles of vaccination. However, during the fourth cycle, the patient requested to be removed from the study. Melanoma patient NW886 developed stabilization of a lung metastasis during three cycles of vaccination, and vaccination is being continued.
In the five NY-ESO-1 antibody-positive patients, two patients (NW516, NW889) experienced progressive disease, and three patients (NW731, NW903, NW924) showed disease stabilization during two cycles of vaccination. In the two patients with disease progression, treatment was discontinued after one vaccine cycle because of development of a brain metastasis (NW516) or extensive bilateral pleural effusion (NW889). In patients with a disease response, patient NW731 had a complete regression of two s.c. metastases and stabilization of two others. A new s.c. lesion appeared after three cycles of immunization, and the patient was removed from the protocol and underwent surgery. Patient NW903 had stabilization of s.c. and lymph node metastases during three cycles of vaccination, but then developed intraspinal metastases and progression of lymph node metastases. Immunization was discontinued, and radiotherapy of spinal disease was initiated. Patient NW924 had a partial regression of s.c. metastases and no measurable changes of three other lesions throughout four cycles of vaccination, and immunization is being continued.
Discussion
There has been considerable progress over the past several years in identifying peptides derived from human tumor antigens recognized by CD8+ T cells (24, 25). A growing list of peptides with the corresponding MHC class I restriction elements have been identified, e.g., Melan A/MART-1 (5, 26), gp100 (27, 28), tyrosinase (7, 29, 30), and MAGE (2, 3, 31, 32). Because peptides are relatively easy to prepare for clinical use, peptide-based cancer vaccines are being widely tested, either with peptides alone (33, 34) or mixed with an adjuvant (35), administered with a systemic cytokine, such as GM-CSF (36) or IL-12 (37), or presented by dendritic cells (38) or other antigen-presenting cells (39). Experience to date indicates that peptide vaccines can elicit specific CD8+ T-cell responses (33, 34, 40), and that such responses are associated in some cases with tumor regressions or disease stabilization (33). However, the use of different peptides, vaccine strategies, and methods to monitor CD8+ T-cell responses, as well as variables involved in patient selection and response, make it impossible to assess which approach to peptide vaccination is most promising.
The NY-ESO-1 system has several characteristics that should facilitate comparative testing of different vaccine strategies. The high inherent immunogenicity of NY-ESO-1 is particularly striking, with approximately 50% of individuals with advanced NY-ESO-1-expressing tumors having a strong humoral immune response (20). This immune response is clearly antigen driven: removal or regression of NY-ESO-1+ tumors results in loss of antibodies, and patients with small NY-ESO-1+ tumor burdens rarely have detectable antibody (22). The identification of three NY-ESO-1 peptides recognized by cytotoxic T cells in the context of HLA-A2 (21) permitted the development of ELISPOT and tetramer assays for monitoring CD8+ T-cell responses to NY-ESO-1 (23). As recently reported, virtually all patients with humoral immunity to NY-ESO-1 show a strong CD8+ T-cell response to the antigen, and there is a strong concordance in the results of ELISPOT, tetramer, and cytotoxicity assays for CD8+ T-cell reactivity against NY-ESO-1 (23).
Given the strong immunogenicity of NY-ESO-1 and the development of robust methodologies for monitoring humoral and cellular immunity to NY-ESO-1, a clinical trial with the three NY-ESO-1 peptides was initiated. For the first cycle of peptide immunization, the peptide was given alone. In subsequent cycles, GM-CSF was administered at a distant site to act as a nonspecific immunopotentiator for peptide immunization. By using ELISPOT as the primary monitoring assay, there was a clear distinction in the response of seronegative and seropositive patients to the 11- and 9-mer NY-ESO-1 peptides. In seronegative patients, the 11-mer response predominated during the initial stage of immunization, with the 9-mer response developing only after repeated peptide vaccinations. The 11-mer response could be rapid and strong, as with patient NW415, or delayed, as with patient NW745. In the seronegative patients, four (NW415, NW745, NW836, NW886) developed strong 11- and 9-mer responses at some point during vaccination. In contrast to the response of seronegative patients to NY-ESO-1 peptide vaccination, seropositive patients show a prominent 9-mer reactivity both pre- and post-vaccination, whereas the 11-mer response is generally of lower magnitude. In one seropositive patient with low initial 9- and 11-mer reactivity (NW924), vaccination was associated with an increased peptide response, particularly after GM-CSF administration. The basis for the stronger immunogenicity of the 11-mer peptide in seronegative patients is unclear, and we are analyzing whether the 11 mer is more effective than the 9 mer in inducing de novo CD8+ T-cell responses in vitro, possibly because it is also recognized by CD4+ T-helper cells.
Along with ELISPOT assays, the DTH response before and during the vaccination procedure represented the other monitoring assay for this trial. Although DTH tests have been carried out in other peptide vaccination trials, there is no consensus regarding the meaning or value of such tests. A major challenge confronting the use of DTH reactions as a monitoring device for cancer vaccines is standardizing the methodology, e.g., standardized test antigens, test procedures, and test recording. As with ELISPOT assays, the DTH response of seronegative patients was distinct from the response of seropositive patients. Three of five seropositive patients showed positive DTH responses at initial vaccine sites, consistent with preexisting NY-ESO-1 immunity in these individuals. In contrast, seronegative patients developed clear DTH responses only after peptide vaccination, and there was a good correlation between DTH and ELISPOT assays in patients NW415, NW836, and NW886. Consistent with past observations (36), GM-CSF greatly augmented the intensity of DTH reactions in vaccinated patients, both seronegative and seropositive, with some reactions showing intense erythema, induration, and, in certain cases, necrosis.
The significance of the tumor responses seen in early-stage cancer vaccine trials has been difficult to assess. Variability of response in different patients and variability of tumor responses in individual patients are characteristic features of most cancer vaccine trials reported to date. For this reason, the major emphasis in early-stage vaccine trials should be on establishing conditions for inducing optimal immune responses to the immunizing antigen. After this has been accomplished, the relation between vaccine-induced immune responses and tumor response can be analyzed. Although tumor regression is obviously the desired endpoint in patients with established disease, it may be that disease stabilization is a more achievable endpoint at this stage in cancer vaccine development. In the current trial of NY-ESO-1 peptide vaccines, occasional tumor regression and mixed response were noted, but what was most impressive was the stabilization of disease in five of seven of the seronegative patients who developed ELISPOT and DTH reactions after NY-ESO-1 peptide vaccination. However, despite disease stabilization and strong CD8+ T-cell and DTH reactivity to NY-ESO-1, three of the five patients (NW415, NW836, NW46) eventually developed disease progression manifested by the emergence of single metastatic lesions while on study (patient NW745 discontinued participation in the study at his request, and patient NW886 is still on treatment). An argument could be made that patients NW415, NW836, and NW46 should have continued vaccination, but the conditions of the protocol necessitated removing them from the study. One possibility to account for the development of new lesions on a background of stable disease is the emergence of NY-ESO-1 antigen-loss or MHC-loss variants. Because NY-ESO-1 elicits such a strong immune response, a strong immunoselection pressure can be expected to be exerted on tumor cells in patients with NY-ESO-1 immunity, either spontaneous or vaccine induced, and we are investigating the NY-ESO-1 and MHC phenotypes of tumors showing progressive growth in patients with NY-ESO-1 antibody and CD8+ T-cell responses. Patients with preexisting spontaneous NY-ESO-1 antibody might be considered less favorable candidates for NY-ESO-1 vaccination because NY-ESO-1 escape variants may already have been formed. However, there was evidence for disease stabilization in three of the five antibody-positive patients, indicating that vaccination may augment tumor resistance in these patients as well.
Acknowledgments
This work was supported by the Cancer Research Institute and Krebsforschung Rhein Main eingetrogener Verein.
Abbreviations
- CT
cancer-testis
- DTH
delayed-type hypersensitivity
- ELISPOT
enzyme-linked immunospot
- GM-CSF
granulocyte–macrophage colony-stimulating factor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220413497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220413497
References
- 1.Boon T, Old L J. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 2.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 3.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio J J, DePlaen E, Lethe B, Brasseur F, Boon T. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y-T, Scanlan M J, Sahin U, Türeci Ö, Gure A O, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old L J. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulie P G, Brichard V, Van Pel A, Wölfel T, Schneider J, Traversari C, Mattei S, DePlaen E, Lurquin C, Szikora J-P, et al. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Sakaguchi K, Appella E, Yannelli J R, Adema G J, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brichard V, Van Pel A, Wölfel T, Wölfel C, DePlaen E, Lethe B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker A B, Schreurs M W J, deBoer A J, Kawakami Y, Rosenberg S A, Adema G J, Figdor C G. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Rivoltini L, Topalian S L, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulie P G, Lehmann F, Lethe B, Herman J, Lurquin C, Andrawiss M, Boon T. Proc Natl Acad Sci USA. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labrecque S, Naor N, Thomson D, Matlashewski G. Cancer Res. 1993;53:3468–3471. [PubMed] [Google Scholar]
- 12.Soussi T. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 13.Wölfel T, Hauer M, Schneider J, Serrano M, Wölfel C, Klehmann-Hieb E, DePlaen E, Hankeln T, Meyer zum Büschenfelde K-H, Beach D. Science. 1995;269:1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- 14.Cheever M A, Disis M L, Bernhard H, Gralow J R, Hunt S L, Huseby E S, Quin H L, Takahashi M, Chen W. Immunol Rev. 1995;45:33–59. doi: 10.1111/j.1600-065x.1995.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 15.Tindle R W. Curr Opin Immunol. 1996;8:643–650. doi: 10.1016/s0952-7915(96)80080-x. [DOI] [PubMed] [Google Scholar]
- 16.Lennette E T, Winberg G, Yadav M, Enblad G, Klein G. Eur J Cancer. 1995;31:1875–1878. doi: 10.1016/0959-8049(95)00354-l. [DOI] [PubMed] [Google Scholar]
- 17.Robbins P F, El-Gamil M, Kawakami Y, Rosenberg S A. Cancer Res. 1994;54:3124–3126. [PubMed] [Google Scholar]
- 18.Wölfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Büschenfelde K-H, Boon T. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 19.Jäger E, Arand M, Ringhoffer M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Melanoma Res. 1996;6:419–425. doi: 10.1097/00008390-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Stockert E, Jäger E, Chen Y-T, Scanlan M J, Gout I, Karbach J, Knuth A, Old L J. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jäger E, Chen Y-T, Drijfhout J W, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, Old L J, Knuth A. J Exp Med. 1998;187:265–269. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jäger E, Stockert E, Zidianakis Z, Chen Y-T, Karbach J, Jäger D, Arand M, Ritter G, Old L J, Knuth A. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar P R, Lee S Y, Jungbluth A, Jäger D, et al. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg S A. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins P F, Rivoltini L, Yanelli J R, Appella E, Rosenberg S A. J Exp Med. 1994;180:347–351. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X Q, Southwood S, Robbins P F, Sette A, Appella E, Rosenberg S A. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 28.Bakker A B H, Schreurs M W J, Tafazzul G, De Boer A J, Kawakami Y, Adema G J, Figdor C G. Int J Cancer. 1995;62:97–102. doi: 10.1002/ijc.2910620118. [DOI] [PubMed] [Google Scholar]
- 29.Kittlesen D J, Thompson L W, Gulden P H, Skipper J C, Colella T A, Shabanowitz J, Hunt D F, Engelhard V H, Slingluff C L, Jr, Shabanowitz J A. J Immunol. 1998;160:2099–2106. [PubMed] [Google Scholar]
- 30.Brichard V G, Herman J, Van Pel A, Wildmann C, Gaugler B, Wšlfel T, Boon T, Lethe B. Eur J Immunol. 1996;26:224–230. doi: 10.1002/eji.1830260135. [DOI] [PubMed] [Google Scholar]
- 31.Van der Bruggen P, Szikora J-P, Boel P, Wildmann C, Somville M, Sensi M, Boon T. Eur J Immunol. 1994;24:2134–2140. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- 32.Van der Bruggen P, Bastin J, Gajewski T, Coulie P G, Boel P, DeSmet C, Traversari C, Townsend A, Boon T. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 33.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier M-H, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Int J Cancer. 1998;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Jäger E, Bernhard H, Romero P, Ringhoffer M, Arand M, Karbach J, Ilsemann C, Hagedorn M, Knuth A. Int J Cancer. 1996;66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Singh M, O'Hagan D. Nat Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]
- 36.Jäger E, Ringhoffer M, Dienes H-P, Arand M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Rodolfo M, Colombo M P. Methods. 1999;19:114–120. doi: 10.1006/meth.1999.0836. [DOI] [PubMed] [Google Scholar]
- 38.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, et al. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Chakraborty N G, Sporn J R, Kurtzman S H, Ergin M T, Mukherji B. Cancer Res. 1996;56:2479–2484. [PubMed] [Google Scholar]
- 40.Rosenberg S A. J Natl Cancer Inst. 1996;88:1635–1644. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]