Abstract
The hexosamine pathway has been implicated in the pathogenesis of diabetic complications. We determined first that hyperglycemia induced a decrease in glyceraldehyde-3-phosphate dehydrogenase activity in bovine aortic endothelial cells via increased production of mitochondrial superoxide and a concomitant 2.4-fold increase in hexosamine pathway activity. Both decreased glyceraldehyde-3-phosphate dehydrogenase activity and increased hexosamine pathway activity were prevented completely by an inhibitor of electron transport complex II (thenoyltrifluoroacetone), an uncoupler of oxidative phosphorylation (carbonyl cyanide m-chlorophenylhydrazone), a superoxide dismutase mimetic [manganese (III) tetrakis(4-benzoic acid) porphyrin], overexpression of either uncoupling protein 1 or manganese superoxide dismutase, and azaserine, an inhibitor of the rate-limiting enzyme in the hexosamine pathway (glutamine:fructose-6-phosphate amidotransferase). Immunoprecipitation of Sp1 followed by Western blotting with antibodies to O-linked GlcNAc, phosphoserine, and phosphothreonine showed that hyperglycemia increased GlcNAc by 1.7-fold, decreased phosphoserine by 80%, and decreased phosphothreonine by 70%. The same inhibitors prevented all these changes. Hyperglycemia increased expression from a transforming growth factor-β1 promoter luciferase reporter by 2-fold and increased expression from a (−740 to +44) plasminogen activator inhibitor-1 promoter luciferase reporter gene by nearly 3-fold. Inhibition of mitochondrial superoxide production or the glucosamine pathway prevented all these changes. Hyperglycemia increased expression from an 85-bp truncated plasminogen activator inhibitor-1 (PAI-1) promoter luciferase reporter containing two Sp1 sites in a similar fashion (3.8-fold). In contrast, hyperglycemia had no effect when the two Sp1 sites were mutated. Thus, hyperglycemia-induced mitochondrial superoxide overproduction increases hexosamine synthesis and O-glycosylation of Sp1, which activates expression of genes that contribute to the pathogenesis of diabetic complications.
Diabetic hyperglycemia causes a variety of pathologic changes in small vessels, arteries, and peripheral nerves (1, 2). Three major hypotheses about how hyperglycemia causes diabetic complications have generated extensive data as well as several clinical trials based on specific inhibitors of these pathways (3–6). These three pathways—activation of protein kinase C isoforms (7), increased formation of glucose-derived advanced glycation endproducts (3), and increased glucose flux through the aldose reductase pathway (8)—recently have been shown to be consequences of a single common mechanism, hyperglycemia-induced mitochondrial superoxide overproduction (1).
A fourth hypothesis about how hyperglycemia causes diabetic complications has been formulated recently (9, 10), in which glucose is shunted into the hexosamine pathway. Inhibition of the rate-limiting enzyme in the conversion of glucose to glucosamine, glutamine:fructose-6-phosphate amidotransferase, blocks hyperglycemia-induced increases in the transcription of both transforming growth factor-α (9) and transforming growth factor-β1 (TGFβ1; ref. 10). This pathway has been shown previously to play an important role in hyperglycemia-induced and fat-induced insulin resistance (11–13).
The mechanism by which increased flux through the hexosamine pathway mediates hyperglycemia-induced increases in gene transcription is not known, but the observation that Sp1 sites regulate hyperglycemia-induced activation of the PAI-1 promoter in vascular smooth muscle cells (14) suggests that covalent modification of Sp1 by GlcNAc may explain the link between hexosamine pathway activation and hyperglycemia-induced changes in gene transcription. Virtually every RNA polymerase II transcription factor examined has been found to be O-GlcNAcylated (15), and the glycosylated form of Sp1 seems to be more transcriptionally active than the deglycosylated form of the protein (16). A 4-fold increase in Sp1 O-GlcNAcylation caused by inhibition of the enzyme O-GlcNAc-β-N-acetylglucosaminidase resulted in a reciprocal 30% decrease in its level of serine/threonine phosphorylation, supporting the concept that O-GlcNAcylation and phosphorylation compete to modify the same sites on this protein (17).
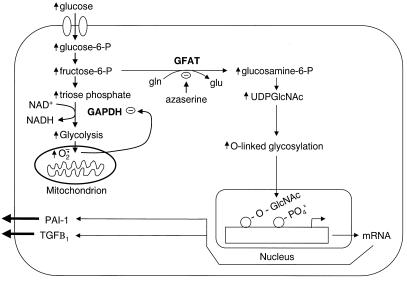
In the present study, we show that hyperglycemia-induced mitochondrial superoxide overproduction inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity and activates the hexosamine pathway, presumably by diverting the upstream metabolite fructose-6-phosphate from glycolysis to glucosamine formation. Hyperglycemia-induced activation of the hexosamine pathway increases O-GlcNAcylation and decreases serine/threonine phosphorylation of the transcription factor Sp1. Hyperglycemia-induced O-GlcNAcylation of Sp1-increased Sp1 transactivation and Sp1-dependent expression of both TGFβ1 and PAI-1 (Fig. 1).
Figure 1.
Schematic mechanism by which hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and increases Sp1-dependent gene expression. Increased glycolysis generates superoxide by increasing the proton electrochemical gradient generated by the mitochondrial electron transport chain (1, 34). Superoxide, thus generated, partially inhibits GAPDH enzymatic activity, diverting fructose-6-phosphate into the hexosamine pathway and increasing UDP-GlcNAc. This increase leads to increased O-GlcNAcylation of Sp1, increased SP1 transactivation, and Sp1-dependent gene expression.
Experimental Procedures
Materials.
Eagle's MEM, nonessential amino acids, and antibiotics were from GIBCO. FBS was from HyClone. Thenoyltrifluoroacetone (TTFA), carbonyl cyanide m-chlorophenylhydrazone (CCCP), azaserine, monoclonal anti-phosphoserine, and anti-phosphothreonine antibodies were purchased from Sigma. Manganese (III) tetrakis(4-benzoic acid) porphyrin (TBAP) was from Calbiochem. Anti-Sp1 IgG was from Santa Cruz Biotechnology. Anti-O-linked GlcNAc (RL2) was from Affinity BioReagents (Golden, CO). Protein A Sepharose was obtained from Amersham Pharmacia.
Adenoviral Vectors.
Rat uncoupling protein 1 (UCP-1) sense and antisense cDNAs were provided by D. Riguier (Centre National de la Recherche Scientifique-Unite Propre 1511, Meudon, France) and human manganese superoxide disumtase (MnSOD) cDNA was provided by L. Oberley (University of Iowa College of Medicine, Iowa City). The cDNAs were cloned into the shuttle vector pAd5CMVK-NpA, and adenoviral vectors were prepared by the Gene Transfer Vector Core at the University of Iowa. Cells were infected 48 h before each experiment with either sense or antisense vectors at a multiplicity of infection of 500 for 90 min. Then, cells were washed and fresh medium was added.
Cell Culture Conditions.
Confluent bovine aortic endothelial cells (BAECs; passage 4–10) were maintained in Eagle's MEM containing 0.4% FBS, essential and nonessential amino acids, and antibiotics. Cells were incubated with 5 mM glucose, 30 mM glucose, 30 mM glucose plus 10 μM TTFA, 0.5 μM CCCP, 100 μM TBAP, or 5 μM azaserine for 48 h, or they were infected with UCP-1 adenovirus, MnSOD adenovirus, or control adenovirus 48 h before addition of 30 mM glucose.
GAPDH Activity.
BAECs were grown to confluency, harvested by using trypsin-EDTA after washing twice with PBS, and resuspended in lysis buffer. The lysate was sonicated on ice. The cytosolic fraction was prepared by centrifuging the lysate at 100,000 × g at 4°C for 30 min. Protein was measured by using the Bio-Rad Coomassie Plus Protein Assay System. Enzyme activity was determined as described (18). Briefly, 1–5 μg of cytosolic protein was added to 1 ml of assay buffer at room temperature, and the OD was measured at 340 nm. The initial measurement time was 10 sec for 1 min and then every minute for 60 min. Activity was expressed as nmol/sec per mg of protein.
UDP-GlcNAc Concentration.
UDP-GlcNAc concentration was determined as described (19). Cells were homogenized in three volumes (≈600 μl) of cold 0.6 M perchloric acid and kept at 0°C for 10 min. The precipitated proteins were removed by centrifugation for 5 min at 13,500 × g, and the supernatant was diluted in four volumes of 0.01 M potassium dihydrogenphosphate, adjusted to pH 2.5 with aqueous 1 M dipotassium hydrogenphosphate, and rediluted with two volumes of 0.01 M potassium dihydrogenphosphate (pH 2.5). Before injection into the HPLC system, the cell extracts were subjected to solid-phase extraction on a 3-ml ion-exchange cartridge (LC-SAX; Supelco) by using aqueous potassium dihydrogenphosphate solutions of increasing molarity: 10 mM (5 ml), 50 mM (2.5 ml), 300 mM (2 ml), pH 2.5 .The 300 mM eluates were divided in fractions of 0.5 ml, and the two most concentrated fractions (containing 80% of the UDP-GlcNAc) were concentrated for subsequent HPLC analysis. Portions (700 μl) of the fractions of UDP-GlcNAc were injected in duplicate into the HPLC system (LC18-T; Supelco), and the UDP-GlcNAc concentrations were determined by UV detection.
Luciferase Assay.
BAECs (1 × 106) were cultured in 6-well plates. Cells were cotransfected with luciferase-reporter plasmids (1 μg/ml) and pRL renilla luciferase plasmids (1 μg/ml) by using GenePorter reagent. Cells were incubated with 5 mM glucose, 30 mM glucose, 30 mM glucose plus 10 μM TTFA, 0.5 μM CCCP, 100 μM TBAP, or 5 μM azaserine for 48 h. Cells were infected with UCP-1 adenovirus, MnSOD adenovirus, or control adenovirus 48 h before luciferase plasmid transfection. Cell lysates were obtained by using 250 μl per well of cell lysis buffer (Promega). Luciferase activity was measured by using 20 μl of cell lysate per assay tube in an Autolumat machine (LB 953; EG&G Berthold, Oak Ridge, TN). Luciferase transfection efficiency was normalized by dual-luciferase analysis by using pRL renilla luciferase control. Reporter constructs included pA835luc (20), containing −835 bp of the TGFβ1 gene promoter; pA3luc (21), containing −744 bp of the PAI-1 promoter; pGluc 85, containing −85 bp of the PAI-1 promoter; and pGluc 85 mSP-1A+B, in which the two Sp1 sites of pGluc 85 were mutated (14). pGluc 85 and pGluc 85 mSP-1A+B were generously provided by Douglas E. Vaughan (Departments of Medicine and Pharmacology, Vanderbilt University, Nashville, TN).
Immunoprecipitation (IP).
BAECs were plated in 100-mm cell culture plates and grown to confluency. Cells (2 × 107) were scraped from the plates, pelleted, and washed twice with cold PBS. The pellet was resuspended in cold lysis buffer (0.5 ml) and incubated on ice for 30 min with gentle vortexing. Cellular debris was pelleted for 20 min at 75,000 × g at 4°C. The supernatants were dialyzed against the binding buffer at 4°C, overnight. Protein (500 μg) was immunoprecipitated with 4 μg of Sp1 antibody (rabbit polyclonal; Santa Cruz Biotechnology) and 20 μl of Protein A Sepharose 4B (Amersham Pharmacia) in binding buffer (final concentration 1 μg of protein per μl), and the samples were rotated overnight at 4°C. The IP complexes were pelleted by centrifugation (1,000 × g) and washed 4 to 5 times with binding buffer. The pellet was resuspended in 1× sample buffer, boiled, and analyzed by SDS/7.5% PAGE with Western blotting.
Western Blotting.
IP Sp1 proteins electrophoresed on 7.5% PAGE gels were transferred onto a nitrocellulose membrane. The immunoblots were developed with a 1:1,000 dilution of affinity-purified monoclonal antibodies against GlcNAc (Affinity BioReagents, Neshanic Station, NJ), phosphoserine, phosphothreonine, or Sp1 (Sigma). The signal was detected with an enhanced chemiluminescence kit according to the manufacturer's instructions (Amersham Phamacia), and the images were scanned into a Molecular Dynamics FluorImager and analyzed by using the IMAGEQUANT 5.5 program.
Statistics.
Data were analyzed by using the one-factor ANOVA procedure to compare the means of all groups. The Tukey–Kramer multiple-comparisons procedure was used to determine which pairs of means were different.
Results
Effect of Hyperglycemia-Induced Mitochondrial Superoxide Overproduction on GAPDH Activity.
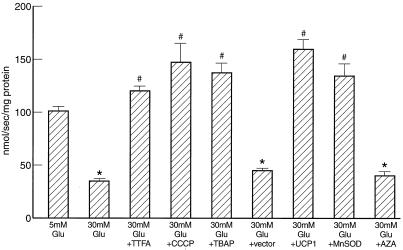
Hyperglycemia induces mitochondrial superoxide overproduction in BAECs (1). Because GAPDH activity is reversibly inhibited by increased reactive oxygen species (22), the effect of 30 mM glucose on GAPDH activity was assessed (Fig. 2). Hyperglycemia reduced GAPDH activity by 66%, from 101.1 ± 4.8 nmol/sec per mg for 5 mM glucose to 34.9 ± 3.4 nmol/sec per mg for 30 mM glucose. Inhibition of mitochondrial superoxide overproduction by TTFA, an inhibitor of complex II; CCCP, an uncoupler of oxidative phosphorylation; or TBAP, a superoxide dismutase mimetic, (1, 23) completely prevented the reduction in GAPDH activity induced by 30 mM glucose. Overexpression of UCP-1 or MnSOD also prevented the effect of hyperglycemia, whereas antisense cDNA in the same gene transfer vector did not. In contrast, azaserine, an inhibitor of the hexosamine pathway, had no effect on GAPDH activity. GAPDH protein levels, as assessed by Western blot, were unaltered by any of the incubation conditions (data not shown).
Figure 2.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction and of a hexosamine pathway inhibitor on GAPDH activity. AZA, azaserine. *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cells incubated in 30 mM glucose. n = 6.
Effect of Hyperglycemia-Induced Mitochondrial Superoxide Overproduction on Hexosamine Pathway Activity.
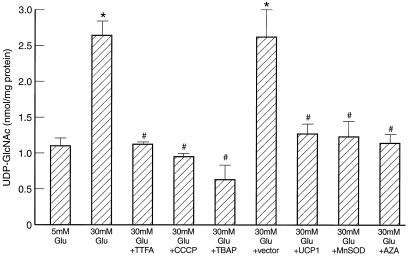
Inhibition of GAPDH activity increases intracellular levels of glyceraldehyde-3-phosphate (22) and thus may increase levels of proximal glycolytic intermediates such as fructose-6-phosphate levels as well. Therefore, the effect of hyperglycemia-induced GAPDH inhibition on hexosamine pathway activity was assessed (Fig. 3). Hyperglycemia induced a 2.5-fold increase in BAEC UDP-GlcNAc levels, from 1.10 ± 0.09 to 2.64 ± 0.19 nmol/mg of protein. Inhibition of mitochondrial superoxide overproduction by TTFA, CCCP, or TBAP completely prevented the increase in UDP-GlcNAc induced by 30 mM glucose. Overexpression of UCP-1 or MnSOD also prevented the effect of hyperglycemia, whereas antisense cDNA in the same gene transfer vector did not. Azaserine (11), a specific inhibitor of the rate-limiting enzyme in the hexosamine pathway glutamine:fructose-6-phosphate amidotransferase, also prevented this increase in UDP-GlcNAc.
Figure 3.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction and azaserine (AZA) on hexosamine pathway activity. *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cells incubated in 30 mM glucose. n = 3.
Effect of Hyperglycemia-Induced Mitochondrial Superoxide Overproduction and Hexosamine Pathway Blockade on Sp1 O-Linked GlcNAc, Phosphoserine, and Phosphothreonine.
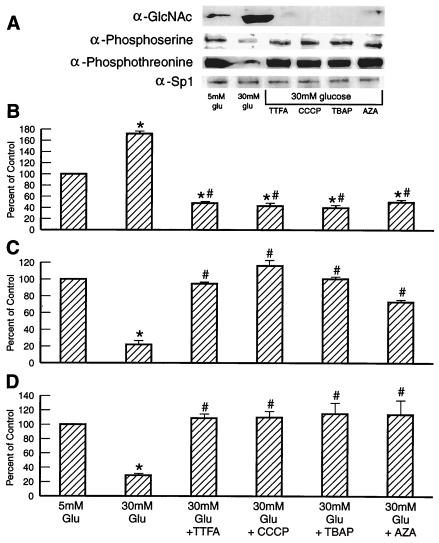
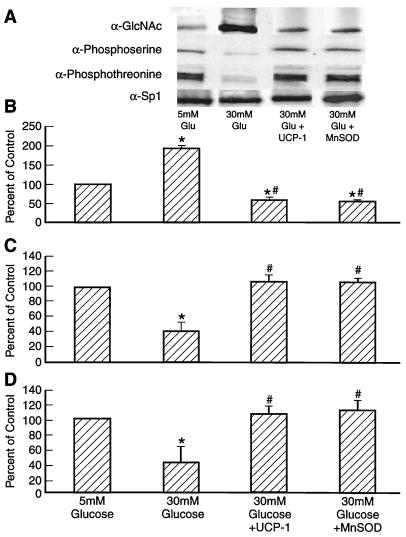
IP of Sp1 followed by Western blotting with antibodies to O-linked GlcNAc, phosphoserine, and phosphothreonine showed that hyperglycemia increased GlcNAc by 1.7-fold, whereas it decreased phosphoserine by 80% and phosphothreonine by 70% (Fig. 4). TTFA, CCCP, TBAP, and azaserine reduced GlcNAc by 95% and restored phosphoserine and phosphothreonine levels to normal. Overexpression of UCP-1 or MnSOD also prevented the effect of hyperglycemia (Fig. 5).
Figure 4.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction and azaserine (AZA) on Sp1 O-linked GlcNAc, phosphoserine, and phosphothreonine. (A) Representative IP-Western blot. (B) Sp1 O-linked GlcNAc (relative densitometric means of three IP-Western blots). (C) Sp1 phosphoserine (relative densitometric means of three IP-Western blots). (D) Sp1 phosphothreonine (relative densitometric means of three IP-Western blots). *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cell incubated in 30 mM glucose. n = 3.
Figure 5.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction by UCP-1 and MnSOD on Sp1 O-linked GlcNAc, phosphoserine, and phosphothreonine. (A) Representative IP-Western blot. (B) Sp1 O-linked GlcNAc (relative densitometric means of three IP-Western blots). (C) Sp1 phosphoserine (relative densitometric means of three IP-Western blots). (D) Sp1 phosphothreonine (relative densitometric means of three IP-Western blots). *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cells incubated in 30 mM glucose. n = 3.
Effect of Hyperglycemia-Induced Mitochondrial Superoxide Overproduction and Hexosamine Pathway Blockade on TGFβ1 and PAI-1 Promoter Activity.
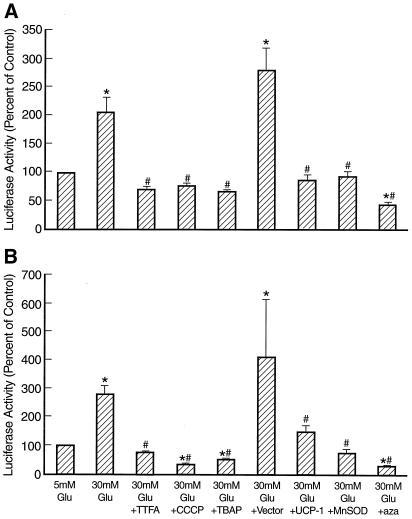
Hyperglycemia increased transcriptional activity of a TGFβ1 promoter construct by 2-fold (Fig. 6A), similar to that reported in murine and porcine mesangial cells (10, 20). Inhibition of mitochondrial superoxide overproduction by TTFA, CCCP, or TBAP completely prevented the increase in TGFβ1 transcription induced by 30 mM glucose (Fig. 6A). Overexpression of UCP-1 or MnSOD also prevented the effect of hyperglycemia, whereas antisense cDNA in the same gene transfer vector did not. Hexosamine pathway blockade by azaserine reduced the level of TGFβ1 transcription in the presence of 30 mM glucose to almost one third of that observed in the presence of 5 mM glucose. Hyperglycemia also increased transcriptional activity of a 744-bp PAI-1 promoter construct by nearly 3-fold (Fig. 6B), similar to that reported in rat aortic smooth muscle cells (14). TTFA, CCCP, TBAP, UCP-1, MnSOD, and azaserine prevented the increase in PAI-1 transcription induced by 30 mM glucose (Fig. 6B). Together, these data suggest that hyperglycemia-induced increases in both TGFβ1 and PAI-1 transcription are mediated by increased hexosamine pathway activity resulting from mitochondrial superoxide overproduction diverting fructose-6-phosphate from glycolysis to the hexosamine pathway.
Figure 6.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction and azaserine (aza) on TGFβ1 (A) and PAI-1 (B) promoter activity. *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cells incubated in 30 mM glucose. n = 3.
Effect of Hyperglycemia-Induced Mitochondrial Superoxide Overproduction and Hexosamine Pathway Blockade on Sp1-Dependent PAI-1 Promoter Activity.
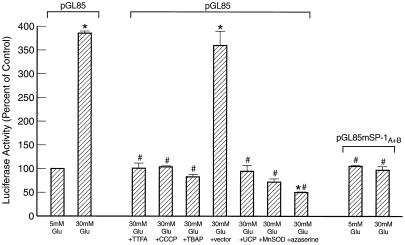
Because the hyperglycemia-responsive element of the PAI-1 promoter has been localized to sequence between −85 and −42 bp containing two Sp1 binding sites (14), the effect of inhibitors of mitochondrial superoxide overproduction and of the hexosamine pathway was determined by using a promoter-deletion construct comprising 85 nucleotides of upstream sequence (pGL85). As shown in Fig. 7, TTFA, CCCP, TBAP, UCP-1, MnSOD, and azaserine inhibited hyperglycemia-induced PAI-1 expression in a manner identical to that observed in Fig. 6B. In contrast, hyperglycemia had no effect when the two Sp1 sites in pGL85 were mutated (pGluc 85 mSP-1A + B). Basal expression from pGluc 85 mSP-1A + B was not changed by any of the inhibitors (data not shown). These data indicate that the increase in PAI-1 expression induced by hyperglycemia reflects changes in Sp1 transactivation mediated by increased hexosamine pathway flux.
Figure 7.
Effect of inhibitors of hyperglycemia-induced mitochondrial superoxide overproduction and azaserine on PAI-1 promoter activity from a truncated promoter containing either two Sp1 binding sites (pGL 85) or two mutated Sp1 binding sites (pGL 85 mSP-1A + B). *, P < 0.01 compared to cells incubated in 5 mM glucose. #, P < 0.01 compared to cells incubated in 30 mM glucose. n = 3.
Discussion
We have recently shown that a single unifying mechanism, increased production of superoxide by the mitochondrial electron transport chain, serves as a causal link between elevated glucose and each of the three major pathways responsible for hyperglycemic damage (1). In this paper, we show that this same mechanism is also responsible for abnormal activation of the hexosamine pathway in BAECs. The observation that hyperglycemia-induced superoxide overproduction inhibits GAPDH activity by 66% suggests that increased levels of the proximal glycolytic intermediate fructose-6-phosphate are diverted into the hexosamine pathway. Hyperglycemia-induced increases in TGFβ1 and PAI-1 promoter activity were prevented both by inhibiting mitochondrial superoxide production and by inhibiting the hexosamine pathway. A previous report had established a connection between hyperglycemia, the hexosamine pathway, and TGFβ1 (10). In contrast, hyperglycemia, but not exogenous glucosamine, increased PAI-1 expression in vascular smooth muscle cells (14). This lack of response to exogenous glucosamine may reflect low transport in that particular cell type. In our system, the observed effect on PAI-1 of increased mitochondrial superoxide production and the resultant increase in hexosamine pathway activity was mediated by changes in Sp1 transactivation. Both increased mitochondrial superoxide production and the resultant increase in hexosamine pathway activity increased modification of Sp1 by GlcNAc and reciprocally decreased Sp1 serine and threonine phosphorylation.
The increased promoter activity resulting from hyperglycemia-induced modification of Sp1 by GlcNAc could reflect effects either on Sp1 binding or on the transcriptional activation function of Sp1. However, DNase I protection studies have shown that GlcNAc has no appreciable affect on the DNA binding activity of Sp1, although GlcNAc-modifed Sp1 is 3- to 5-fold more efficient at activating transcription than unglycosylated Sp1 synthesized in Escherichia coli (24). Under conditions of glucose starvation, forskolin treatment reduces Sp1 binding by increasing the amount of Sp1 that is degraded by the proteosome (25). However, in these experiments, the presence of 5 mM glucose blocked this effect, consistent with the constant amount of Sp1 protein present in the different conditions of our experiments.
The glutamine-rich transactivating domain of Sp1 contains a dominant O-GlcNAc epitope, and this modification was found to block Sp1 protein interactions (26). Because negative regulation of Sp1 transactivation is correlated with the binding of other nuclear proteins to the amino terminus of the transactivation domain (p74 and the retinoblastoma-related protein p107), it seems likely that hyperglycemia-induced increases in GlcNAc modification of Sp1 activate transcription by blocking Sp1 interactions with such repressor proteins (27, 28).
GlcNAc modification of Sp1 may regulate other glucose-responsive genes in addition to TGFβ1 and PAI-1. Glucose-responsive transcription is regulated by Sp1 sites in the acetylCoAcarboxylase gene, the rate-limiting enzyme for fatty acid synthesis, for example, and it seems that posttranslational modification of Sp1 is responsible for this effect (29, 30). The l-pyruvate kinase gene, in contrast, contains an E box motif carbohydrate response element with two 5′-CACGTG motifs separated by 5 bp. However, synergy is required with additional nuclear factor binding sites to give a functional response (31–33). Because virtually every RNA polymerase II transcription factor examined has been found to be O-GlcNAcylated (15), it is possible that reciprocal modification by O-GlcNAcylation and phosphorylation of transcription factors other than Sp1 may function as a more generalized mechanism for regulating glucose-responsive gene transcription.
In addition to transcription factors, many other nuclear and cytoplasmic proteins are dynamically modified by O-GlcNAc moieties, and may exhibit reciprocal modification by phosphorylation in a manner analagous to Sp1 (15). Thus, activation of the hexosamine pathway by hyperglycemia-induced mitochondrial superoxide overproduction may result in many changes in both gene expression and in protein function that contribute to the pathogenesis of diabetic complications.
Acknowledgments
This work was supported by National Institutes of Health Grant DK-33861. The University of Iowa Gene Transfer Vector Core is supported in part by the National Institutes of Health and the Roy J. Carver Foundation.
Abbreviations
- PAI-1
plasminogen activator inhibitor-1
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- TTFA
thenoyltrifluoroacetone
- TGFβ1
transforming growth factor-β1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- TBAP
manganese (III) tetrakis(4-benzoic acid) porphyrin
- MnSOD
manganese superoxide dismutase
- BAECs
bovine aortic endothelial cells
- IP
immunoprecipitation
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nishikawa T, Edelstein D, Du X L, Yamagishi S I, Matsumura T, Kaneda Y, Yorek M A, Beebe D, Oates P J, Hammes H-P, et al. Nature (London) 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 2.National Diabetes Data Group. Diabetes in America. 2nd Ed. National Institute of Health, Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1994. [Google Scholar]
- 3.Brownlee M. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 4.Ishii H, Jirousek M R, Koya D, Takagi C, Xia P, Clermont A, Bursel S E, Kern T S, Ballas L M, Heath W F, et al. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 5.Park L, Raman K G, Lee K J, Lu Y, Ferran L J, Jr, Chow W S, Stern D, Schmidt A M. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 6.Sima A A, Prashar A, Zhang W X, Chakrabarti S, Greene D A. J Clin Invest. 1990;85:1410–1420. doi: 10.1172/JCI114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koya D, King G L. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 8.Lee A Y, Chung S K, Chung S S. Proc Natl Acad Sci USA. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayeski P P, Kudlow J E. J Biol Chem. 1996;271:15237–15243. doi: 10.1074/jbc.271.25.15237. [DOI] [PubMed] [Google Scholar]
- 10.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher E D. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall S, Bacote V, Traxinger R R. J Biol Chem. 1991;5:4706–4712. [PubMed] [Google Scholar]
- 12.Rossetti L, Hawkins M, Chen W, Gindi J, Barzilai N. J Clin Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. J Clin Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y Q, Su M, Walia R R, Hao Q, Covington J W, Vaughan D E. J Biol Chem. 1998;273:8225–8231. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 15.Hart G W. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 16.Kadonaga J T, Courey A J, Ladika J, Tjian R. Science. 1988;242:1566–1570. doi: 10.1126/science.3059495. [DOI] [PubMed] [Google Scholar]
- 17.Haltiwanger R S, Grove K, Philipsberg G A. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y, Howlett A, Klein C. Eur J Biochem. 1994;224:447–454. doi: 10.1111/j.1432-1033.1994.00447.x. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti L, Hu M. J Clin Invest. 1993;92:2963–2974. doi: 10.1172/JCI116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman B B, Sharma K, Zhu Y, Ziyadeh F N. Kidney Int. 1998;54:1107–1116. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 21.Mucsi I, Skorecki K L, Goldberg H J. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 22.Knight R J, Kofoed K F, Schelbert H R, Buxton D B. Cardiovasc Res. 1996;32:1016–1023. doi: 10.1016/s0008-6363(96)00137-x. [DOI] [PubMed] [Google Scholar]
- 23.Faulkner K M, Liochev S I, Fridovich I. J Biol Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- 24.Jackson S P, Tjian R. Cell. 1988;55:125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- 25.Han I, Kudlow J E. Mol Cell Biol. 1997;17:2550–2558. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roos M D, Su K, Baker J R, Kudlow J E. Mol Cell Biol. 1997;17:6472–6480. doi: 10.1128/mcb.17.11.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata Y, Kim H G, Rogers K T, Udvadia A J, Horowitz J M. J Biol Chem. 1994;269:20674–20681. [PubMed] [Google Scholar]
- 28.Datta P K, Raychaudhuri P, Bagchi S. Mol Cell Biol. 1995;15:5444–5452. doi: 10.1128/mcb.15.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samira D, Kim K H. J Biol Chem. 1996;271:1385–1392. doi: 10.1074/jbc.271.3.1385. [DOI] [PubMed] [Google Scholar]
- 30.Samira D, Zhang S, DePaoli-Roach A A, Kim J H. J Biol Chem. 1996;271:14692–14697. doi: 10.1074/jbc.271.25.14692. [DOI] [PubMed] [Google Scholar]
- 31.Shih H M, Liu Z, Towle H C. J Biol Chem. 1995;270:21991–21997. doi: 10.1074/jbc.270.37.21991. [DOI] [PubMed] [Google Scholar]
- 32.Shih H M, Towle H C. J Biol Chem. 1992;267:13222–13228. [PubMed] [Google Scholar]
- 33.Shih H M, Towle H C. J Biol Chem. 1994;269:9380–9387. [PubMed] [Google Scholar]
- 34.Korshunov S S, Skulachev V P, Starkov A A. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]