Abstract
Previous work has identified sphingosine kinase-1 (SK1) as a substrate for the cysteine protease cathepsin B in vitro. In this study, the mechanism of SK1 cleavage by cathepsin B was investigated. We identified two initial cleavage sites for the protease, the first at histidine 122 and the second at arginine 199. Mutation analysis showed that replacement of histidine 122 with a tyrosine maintained the activity of SK1 while significantly reducing cleavage by cathepsin B at the initial cleavage site. The efficacy of cleavage of SK1 at arginine 199, however, was not affected. These studies demonstrate that SK1 is cleaved by cathepsin B in a sequential manner after basic amino acids, and that the initial cleavages at the two identified sites occur independently of each other.
Keywords: Sphingosine kinase, cathepsin B, proteases
Introduction
Cathepsins constitute a family of proteases comprised of aspartate proteases, such as cathepsins D and E, serine proteases, such as cathepsins A and G, and cysteine proteases, such as cathepsins B, C, H, K, L, S, and T [1]. Most cathepsin enzymes are endopeptidases, with a few, such as cathepsin B, that can also act as carboxypeptidases [2,3]. Cathepsins B, D, and L are the most abundant lysosomal proteases, having concentrations as high as 1 mM [4]. They are ubiquitous enzymes whose functions have been proposed to extend beyond the confines of the lysosome. In fact, administration of cathepsin B and cathepsin D inhibitors in rats did not result in endogenous protein accumulation in the lysosomes or impaired degradation of exogenous protein [5], suggesting that the function of these proteases in intralysosomal protein degradation is not essential, and that their activities may be important when they are localized extralysosomally under physiological or pathological conditions.
Several studies have proposed crucial extralysosomal functions for cathepsin B. In addition to being localized in the lysosome, cathepsin B (or alternatively spliced variants of cathepsin B) can be found on the surface or in the periplasmic space of tumor cells [6–11], where it can promote invasion. In the mitochondria [12], cathepsin B can mediate nuclear fragmentation. Cathepsin B has also been reported in other cellular compartments [13]. The role of cathepsin B in apoptosis has recently grown with the emerging evidence implicating the lysosome as a critical mediator of programmed cell death [14]. Under conditions where lysosomes become disrupted, cathepsin B is released into the cytosol where it mediates events such as Bid cleavage [15,16] and cytochrome c release [17]. Therefore, the localization of cathepsin B is an important determinant of the effects it produces.
Sphingosine kinase-1 (SK1) is a key enzyme in sphingolipid metabolism that regulates the levels of the bioactive lipids ceramide, sphingosine, and sphingosine-1-phosphate (S1P). Overexpression of SK1 enhances DNA synthesis, increases growth, and inhibits apoptosis in response to several death-inducing agents, including Tumor Necrosis Factor (TNF), ceramide, and genotoxic stress [18,19]. Activation of SK1 by agonist stimulation or overexpression has been shown to attenuate caspase activation and the mitochondrial pathway of cell death [18,19]. The regulation of SK1 by proteases, however, has not been investigated.
Recently, we have shown that cathepsin B and lysosomes colocalize with SK1 in MCF-7 breast cancer cells [20]. More importantly, Tumor Necrosis Factor induced the disruption of the lysosome, release of active cathepsin B into the cytosol, and loss of SK1, an event inhibited by knocking down cathepsin B protein. Moreover, SK1 appeared to be a substrate for cathepsin B in vitro [20].
In this study, we extend these observations to define the multiplecleavage sites for cathepsin B in SK1. Two initial cleavage sites were identified, with one site appearing before the other. Moreover, mutation in the initial site significantly attenuated cleavage by cathepsin B at that site while maintaining SK1 activity.
Materials and Methods
Chemicals and Reagents
Recombinant human liver cathepsin B was from Calbiochem. The rabbit polyclonal anti-SK1 antibody has been described previously [21]. Recombinant SK1 was a kind gift from Dr. Wonhwa Cho (University of Illinois at Chicago). The C-terminus directed SK1 antibody preparation has been described previously [21].
Cell Culture
MCF-7 cells were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, and 100 ug/ml streptomycin. Hela and A549 cells were grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 ug/ml streptomycin.
Site-Directed Mutagenesis
SK1 point mutants were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). Primers used were: N121E (Fwd: 5’-GCT GGC AGC TTC CTT GGA GCA TTA TGC TGG CTA TGA-3’, Rev: 5’-TCA TAG CCA GCA TAA TGC TCC AAG GAA GCT GCC AGC-3), H121E (Fwd: 5’- GGC AGC TTC CTT GAA CGA GTA TGC TGG CTA TGA GCA-3’, Rev: 5’- TGC TCA TAG CCA GCA TAC TCG TTC AAG GAA GCT GCC-3’), H122A (Fwd: GGC AGC TTC CTT GAA CGC ATA TGC TGG CTA TGA GCA GG-3’, Rev: 5’-CCT GCT CAT AGC CAG CAT ATG CGT TCA AGG AAG CTG CC-3’), Y123A (Fwd: 5’-CAG CTT CCT TGA ACC ATG CAG CTG GCT ATG AGC AGG TC-3’, Rev: 5’- GAC CTG CTC ATA GCC AGC TGC ATG GTT CAA GGA AGC TG-3’), H122Y (Fwd: 5’- GCA GCT TCC TTG AAC TAT TAT GCT GGC TAT GAG C-3’, Rev: 5’- GCT CAT AGC CAG CAT AAT AGT TCA AGG AAG CTG C-3’). Mutants were confirmed by DNA sequencing.
Transient Transfection
MCF-7 or Hela cells were seeded in 10 cm dishes and transfected with pcDNA-DEST40-SK1 wildtype or mutant using the Effectene transfection reagent (Qiagen). For MCF-7 cells, 2.0 μg DNA, 500 μl EC buffer, 16 μl Enhancer, and 20 μl Effectene were used. For Hela cells, 1.0 ug DNA, 500 ul EC buffer, 8.0 ul Enhancer, and 10 ul Effectene were used. The cells were analyzed 24–30 h after transfection. A549 cells in 60 mm dishes were transfected with the Effectene transfection reagent; 2.0 ug DNA, 150 ul EC Buffer, 16 ul Enhancer, and 20 ul Effectene were used. Sixteen hours after transfection, the cells were washed with PBS, and fresh growth media was added. Transfectants were analyzed 24 h later.
In vitro Cleavage Assays of SK1 by Cathepsin B
Transfected cells were harvested by scraping in PBS, and lysed on ice for 30 min in Buffer L (25 mM HEPES pH 7.4, 5 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, 5 mM DTT). The lysate was homogenized with a 21 G1 needle and centrifuged at 18000 x g for 30 min. For the cathepsin B assay, 1.0 ul of recombinant SK1 (2 ug/ul) or 10–15 ug of the supernatant proteins were diluted to 4.0 ul in Buffer L and then added to 5 ul of 3X cathepsin B reaction buffer (150 mM sodium acetate pH 6.0, 12 mM EDTA, 24 mM DTT) and 5.0 ul of recombinant cathepsin B to achieve the final concentrations required for the assay. Whenever required, cathepsin B was diluted in 50 mM sodium acetate pH 5.0, 1 mM EDTA prior to its use in the assay. The final volume was brought to 15 ul with ddH20. The cleavage assays were performed by incubation at 37°C for the times indicated in the text. Following incubation, 15 ul of 2X SDS-Laemmli sample buffer was added to the samples; they were boiled, and subjected to SDS-PAGE. The proteins were transferred to Problott PVDF membrane (Applied Biosystems) when Coomassie Brilliant Blue staining was required or to nitrocellulose membranes for Western blotting.
Edman Degradation
The in vitro cleavage assay was performed as described above but the reaction components were multiplied by three in order to obtain enough protein for sequencing. Once transfer was done onto Problott PVDF membranes, the membrane was stained with Coomassie Brilliant Blue until the bands were visible, then destained with 50% methanol and left to dry at room temperature. Edman sequencing was performed by the Protein Sequencing and Peptide Synthesis Facility at the Medical University of South Carolina and by the Microchemical and Proteomics Facility at Emory University.
Sphingosine Kinase Assay
The assay was performed as described in [22] with slight modifications. Briefly, the cells were collected by centrifugation at 3000 x g for 5 min. They were then resuspended in lysis buffer (20 mM Tris-Cl pH 7.4, 1 mM EDTA, 0.5 mM Deoxypyridoxine, 15 mM NaF, 1 mM β-mercaptoethanol, 1 mM sodium orthovanadate, 40 mM β-glycerophosphate, 10% glycerol, 0.5% Triton X-100, 1.5 mM semicarbazide, supplemented with Complete Protease Inhibitor (Roche)). The cell lysates were sonicated, centrifuged at 2500 x g for 15 min and protein concentration was determined on the supernatant. Equal amounts of protein were then incubated with 50 μM sphingosine (delivered in 4 mg/ml fatty acid free BSA) and 1 mM ATP (5–10 μCi, dissolved in 200 mM MgCl2). The reaction was carried out for 30 minutes at 37°C. It was stopped by the addition of 20 μl of 1N HCl and 800 μl chloroform/methanol/HCl (100:200:1). After letting the samples sit at room temperature for 10 minutes, 240 μl of chloroform and 240 μl of 2 M potassium chloride were added, and the samples were centrifuged at 3000 x g for 5 min. The aqueous layer was aspirated, and 250 μl of the organic layer were transferred to new glass tubes. The samples were dried down in a speed vac and then resuspended in chloroform/methanol/HCl (100:200:1). Lipids were resolved on silica TLC plates (Whatmann) using 1-Butanol/methanol/acetic acid/water (80:20:10:20) as a solvent system. Labeled S1P was visualized by autoradiography and quantified by phosphoimager or by scraping and counting in a scintillation counter.
Results
Sequential in vitro Cleavage of SK1 by Cathepsin B
We previously showed that cathepsin B cleaves SK1 in vitro to generate multiple cleavage fragments: one band at ~31 kDa, one band at ~23 kDa, two bands at ~18 kDa, and one band at ~13 kDa [23]. In order to determine the pattern in which these fragments were generated, a time course with 5-fold lower concentration of cathepsin B was performed (Fig. 1A). Incubation of SK1 with 1.3 munits/ul (0.132 uM) of the protease showed that the first major bands to appear were the 31 kDa and the 23 kDa fragments. The detection of these two bands suggests that there may be two initial sites where cathepsin B cleaves SK1. To determine the position of these sites, a Western blot was performed on the in vitro cleavage assay products and the membrane was probed with the SK1 polyclonal antibody directed against the C-terminus of the protein (Fig. 1B). Using 0.3 units/ul of cathepsin B, the first prominent band to be detected by the antibody had a molecular weight of ~31 kDa. At 3.3 munits/ul of the protease, three other bands were clearly seen: one band approximately 23 kDa, one around 21 kDa and one at about 18 kDa. A time course analysis using recombinant SK1 as a substrate showed that the 31 and the 23 kDa bands appeared about the same time (Fig. 1C). The 18 kDa band appeared later (Fig. 1C). These results suggest that cathepsin B cleaves SK1 in a sequential manner with 2 initial cleavage sites.
Fig. 1. In vitro cleavage of SK1 by cathepsin B.
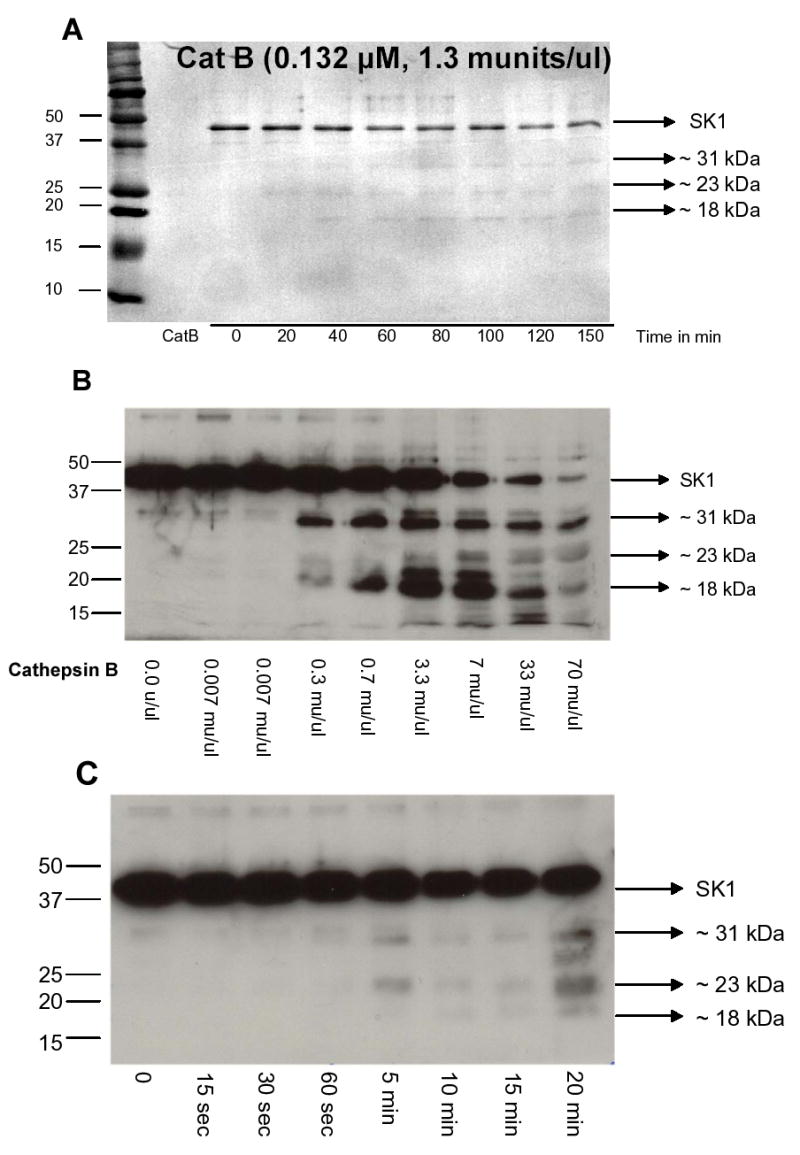
(A) Recombinant SK1 was incubated with recombinant cathepsin B for various times in a cleavage assay as described in “Materials and Methods”. The products of the reaction were run on an SDS-PAGE, transferred to PVDF membrane and stained with Coomassie Brilliant Blue. Several cleavage fragments were identified. (B) Dose response of recombinant cathepsin B incubated with 500 ng of recombinant SK1. The products of the cleavage reaction were analyzed by Western blotting using an SK1 antibody. (C) Time course of recombinant cathepsin B (1.3 munits/ul) incubated with 500 ng of recombinant SK1. The products of the cleavage reaction were analyzed by Western blotting using an SK1 antibody.
Identification of Cathepsin B Cleavage Sites in SK1
To examine whether the cleavage bands on the Coomassie stained membranes were the same as those on the Western blot, we probed the Coomassie membrane from figure 1A with the SK1 antibody. Figure 2A shows that both Coomassie and SK1 probed membranes generated the same pattern of bands: one 31 kDa band and two close 23 kDa bands. The 18 kDa bands on both membranes did not overlap exactly; the band on the Coomassie stained gel was slightly lighter, suggesting that this band does not contain the C-terminus of the SK1 protein [21]. Next, we elected to run the same experiment on a bigger polyacrylamide gel in order to obtain better separation and resolution of the bands. As shown in Fig. 2B, the 31 kDa band matched on the Coomassie and the probed membranes. A doublet at ~23 kDa could also be clearly seen on the blotted membrane. For this doublet, the lower band, which was more intense, matched a band on the Coomassie stained membrane. Two other SK1 bands, one less than 20 kDa and the other less than 15 kDa, were also observed on the Western blot but did not match any bands on the stained membrane. These results indicate that the two initial cleavage fragments generated by cathepsin B action on SK1 retain the C-terminal portion of the protein. Moreover, since the 23 kDa and the 31 kDa bands were both detected by the SK1 antibody, this suggests that the 23 kDa band is an N-terminal truncated derivative of the 31 kDa band.
Fig. 2. Alignment of Coomassie membranes with Western blots.
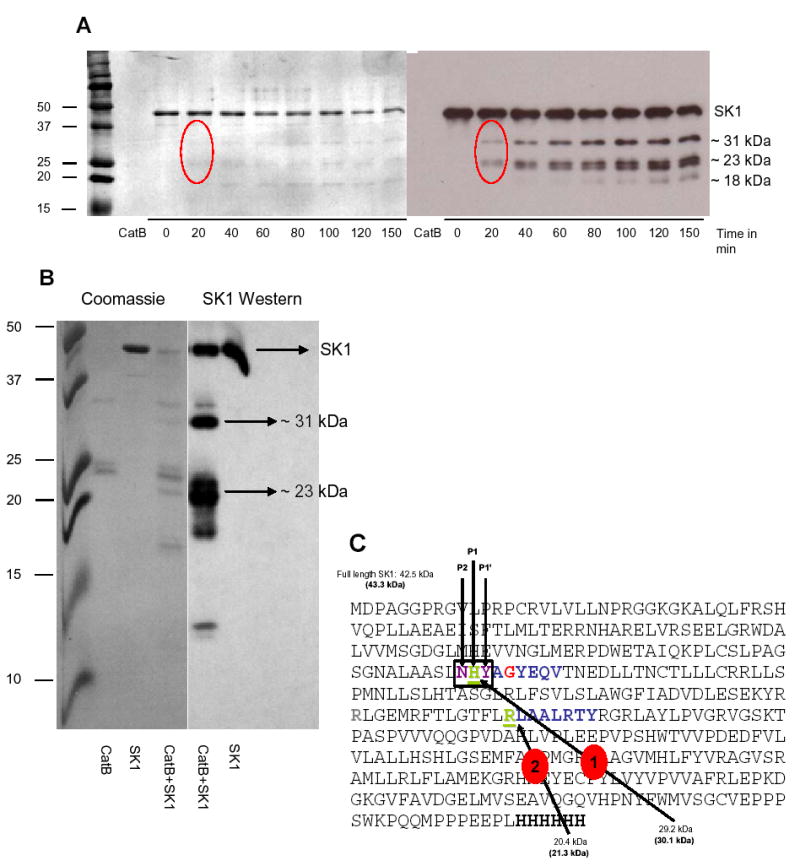
(A and B) Recombinant SK1 was incubated with recombinant cathepsin B and a cleavage assay was performed as described in “Materials and Methods”. The products of the reaction were run on an SDS-PAGE, transferred to PVDF membrane and stained with Coomassie Brilliant Blue or probed for SK1 by Western blotting. The stained and the probed membranes were then aligned to compare the sizes of the cleavage products detected by the two approaches. (C) SK1 sequence showing the cleavage sites identified by Edman degradation; first site after H122 (green) and second one after R199 (green). Expected molecular weights of fragments generated at H122 and R199 are also indicated. Weights in parentheses represent the mass of His-tagged recombinant SK1, which has 6 extra histidine residues at its C-terminus (Bold). The P1 (green), P2 (purple), and P1’ (purple) amino acids at the first cleavage site are also indicated. Amino acids in blue are the residues obtained by Edman sequencing; red amino acids were not detected by Edman sequencing.
Next, the sites of cleavage for cathepsin B were determined. To this end, the 31 kDa and the 23 kDa bands were excised from the Problott PVDF membrane and subjected to Edman degradation for the determination of the amino acid sequences. The 31 kDa band generated the sequence: XAXYEQV and the 23 kDa band the sequence LAALRTY. Examining the SK1 amino acid sequences suggested that the exact cleavage sites are at the H122-Y123 and the R199-L200 peptide bonds (Fig. 2C). Calculating the molecular weights for fragments extending from these cleavage sites to the C-terminus of recombinant SK1 (which has a 6X His tag at the C-terminus) generated bands with molecular weights of 30.1 kDa and 21.3 kDa respectively, which closely match the sizes obtained on the Western blotted membranes. From here on, the 31 kDa and the 23 kDa bands are renamed as 30 kDa and 21 kDa bands, respectively.
Mutation of the P1, P2, or P1’ Amino Acids at the First Cleavage site Reduces Cleavage by Cathepsin B at that Site
It has been previously proposed that the three sites P1, P2, and P1’ are the major amino acids determining cleavage specificity by cathepsin B [24]. The P1 site is the amino acid after which the protease cleaves, the P2 site is the amino acid upstream of P1, and the P1’ is the amino acid downstream of P1 (Fig. 2C). Because the 30 kDa band was the most prominent cleavage fragment and the first to appear, we generated several SK1 point mutants at these three amino acids in the presumed first cleavage site and examined their sensitivities to cleavage by the protease. Incubation of cell lysates transfected with wildtype or point mutants of SK1 with recombinant cathepsin B showed that all were susceptible to cleavage (Fig. 3A) to degrees ranging from 30% in the H122A mutant to 62% in the N121E mutant (Fig. 3B). Differences were also observed in the ratio of the 30 kDa band to full length SK1, which represents the efficiency of cleavage at the P1 amino acid of the first cleavage site. The cleavage efficiency of the wildtype was set at 100% and all mutants were compared to it (Fig. 3C). Interestingly, in all the mutants tested, the cleavage at the P1 site was lower than the wildtype. Mutations of the P1 histidine to an alanine or a glutamate both appeared to be the most effective in reducing cleavage (up to 25%), followed by the P2 and the P1’ mutants, where reduction was by 18% (Fig. 3C). These data suggest that the P1 histidine, followed by the P1’ tyrosine and the P2 asparagine are important determinants of SK1 cleavage by cathepsin B at the P1 position.
Fig. 3. In vitro cleavage of SK1 mutants by cathepsin B.
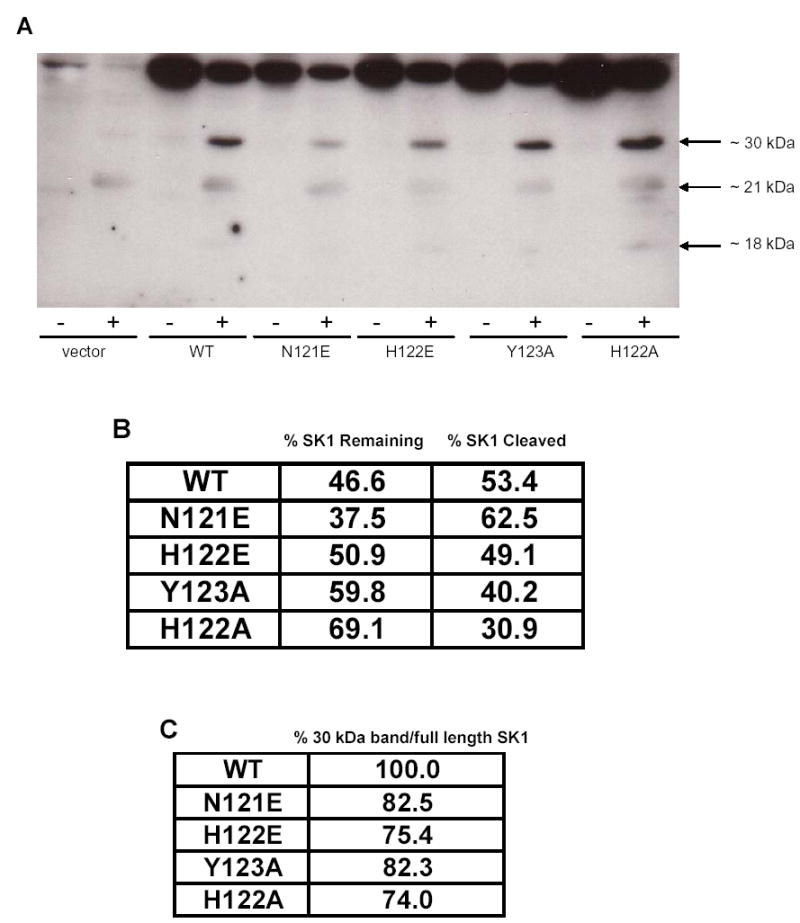
(A) Cleavage assay was performed by incubating recombinant cathepsin B with lysates from MCF-7 cells overexpressing wildtype or mutant SK1. Products were analyzed by Western blotting for SK1. (B) Densitometric quantification of the percentage cleavage of wildtype or mutant SK1 by cathepsin B. (C) Densitometric quantification of cleavage at the P1 amino acid residue of the first cleavage site of SK1 or its mutants. Comparisons were made to wildtype, which was set at 100%.
Mutation of the P1, P2, or P1’ Amino Acids at the First Cleavage Site Reduces SK1 Activity
Next, it was important to determine whether the mutations in SK1 affected the activity of the enzyme. To this end, the various mutants were transfected into cells and their activities were checked by an in vitro SK assay. Each activity was normalized to its protein expression to control for variations in expression. As shown in Fig. 4, all the mutants had lower activity than the wildtype. In particular, the glutamate mutations significantly inhibited the enzyme activity by 80%, suggesting that the introduction of a negative charge in this region of SK1 affects its catalysis (Vmax). The alanine mutations also reduced the SK1 activity by 40% (Y123A) and 60% (H122A). Therefore, despite the ability to reduce cleavage at the P1 site, none of these mutants had an activity that was comparable to wildtype. From these results, it was not possible to determine whether the decrease in cleavage at the P1 site was due to general misfolding of the protein (which also reduced activity) or due to the mutation itself.
Fig. 4. SK assay on SK1 mutants.
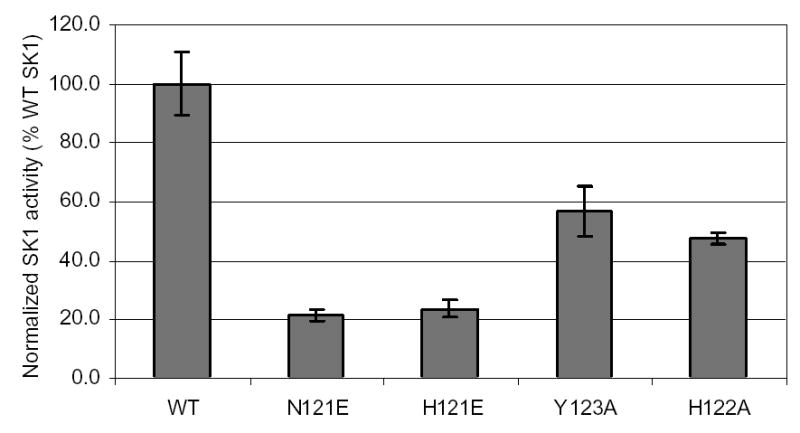
SK assay was performed on cell lysates of MCF-7 cells transfected with wildtype or mutant SK1 proteins. Activity was normalized to SK1 protein expression (Fig. 3) and reported as a percentage relative to wildtype activity, which was set at 100%.
An SK1 P1 Tyrosine Mutant is Strongly Resistant to Cleavage by Cathepsin B at the First Cleavage Site
It became important to identify a mutant that can reduce cleavage at the first cleavage site without compromising SK1 activity. To address this, we aligned SK1 proteins from different species and compared their P1, P2, and P1’ amino acids. The human, mouse and rat SK1s all have identical residues at the P1’ and the P2 positions (Fig. 5). The P1 residue, however, was different in the rat SK1 where the histidine was replaced by a tyrosine (Fig. 5). Therefore, we reasoned that a mutation of the P1-histidine to a tyrosine would unlikely inhibit activity of SK1, yet might change its cleavage susceptibility. Indeed, when SK1 activity in H122Y transfected cells was compared to wildtype, no detectable differences were observed, indicating that this mutant is fully functional (Data not shown).
Fig. 5. ClustalW alignment of a region of human, mouse, and rat SK1 proteins.
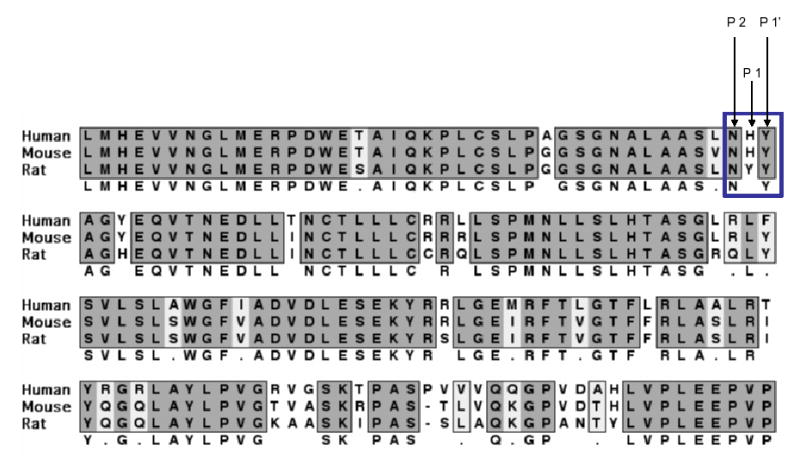
The P1, P2, and P1’ amino acid residues at the first cleavage site from all three species are indicated.
The susceptibility of H122Y to cleavage by cathepsin B was then tested. The mutant showed no rescue from overall cleavage of the full length enzyme (Fig. 6A and Fig. 6B). However, when the ratio of the 30 kDa fragment to full length SK1 (efficiency of cleavage at the first cleavage site) was compared in the H122Y and the wildtype sample, a 70% reduction in cleavage efficiency was observed (Fig. 6C). Interestingly, cleavage at R199 was not reduced (possibly enhanced) (Fig. 6A). These results suggest that the decrease in cleavage efficacy of SK1 by cathepsin B at the first cleavage site is a direct consequence of the mutation rather than a change in enzyme folding (since activity was unchanged). The data also suggest that the cleavage at first site may not be necessary for cleavage at other sites such as R199, since reduction of cleavage at the first site neither affected the decrease in full length SK1 after cathepsin B nor the generation of the 21 kDa cleavage fragments. Interestingly, the 18 kDa fragment, however, was also significantly reduced in the H122Y mutant, suggesting that this fragment may be a cleavage product of the 30 kDa fragment.
Fig. 6. SK1 (H122Y) activity and cleavage.
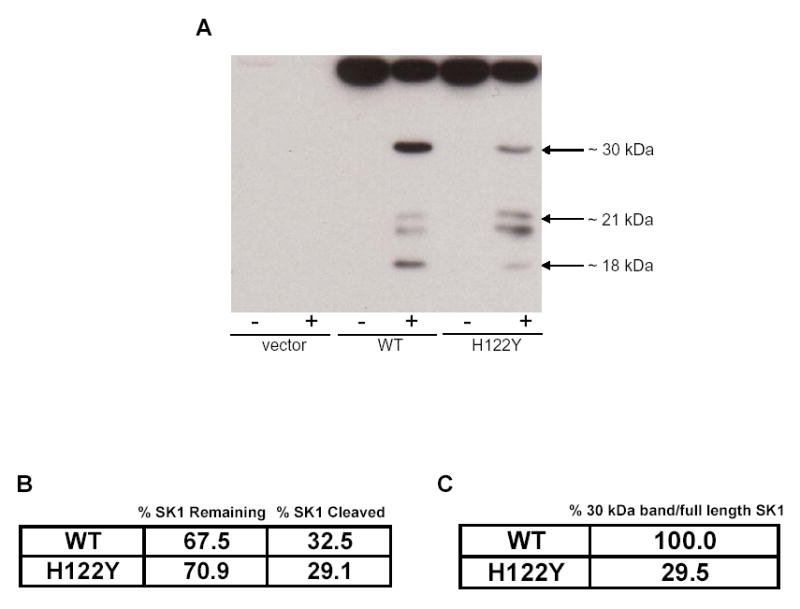
(A) Cleavage assay was performed by incubating recombinant cathepsin B with lysates from Hela cells overexpressing wildtype or mutant SK1. Products were analyzed by Western blotting for SK1. (B) Densitometric quantification of the percentage cleavage of wildtype or H122Y SK1 by cathepsin B. (C) Densitometric quantification of cleavage at the P1 amino acid of the first cleavage site of wildtype or H122Y SK1. Comparisons were made to wildtype, which was set at 100%.
Discussion
In this study, we examined the mechanism of SK1 regulation by cathepsin B in vitro. The cysteine protease cleaved SK1 at multiple sites with two initial sites at histidine 122 and arginine 199. Cathepsin B is a cysteine protease that acts as an exopeptidase at pH<5.5 and as an endopeptidase at pH>5.5 [3]. Its cleavage specificity prefers, but is not restricted to, basic amino acids at the P1 position and hydrophobic or arginine residues at the P2 position [24]. The assays in this study were performed at pH ~ 6.0, supporting the endopeptidase activity of cathepsin B. The P1 residues identified at the two cleavage sites (H122 and R199) are basic amino acids, consistent with the preference of the enzyme as well.
An H122Y SK1 mutant showed significant reduction of cleavage at the first site but did not affect cleavage at the R199 site or total cleavage of full length SK1. These results lead to several conclusions. First, cleavage at H122 is not required for cleavage at R199, and cleavages at the two sites are apparently independent of each other. Second, cathepsin B cleaves SK1 very efficiently, such that mutation at one of the cleavage sites (H122) does not rescue cleavage of the full length enzyme. Therefore, it may be important to mutate more than one cathepsin B cleavage site to obtain an SK1 resistant to cleavage, or to identify the binding site for cathepsin B in SK1 to inhibit the effect of the protease on the enzyme. Moreover, cleavage sites for cathepsin B have been identified in Bid at positions where the protein is flexible [15,16]; hence it is possible to speculate that H122 and R199 in SK1 occupy regions of the protein with fairly high mobilities, which allow them to insert into the active site of cathepsin B, and point mutations, although reduce the efficiency of binding, may not completely abolish cleavage because of flexibility of these regions.
Although we have sequenced two sites of cleavage by Edman degradation, Coomassie stained gels and Western blots in figure 2 showed that there were two cleavage bands with molecular weights close to 20 kDa. While the lower band corresponded to cleavage at R199 and generated a band of 20–21 kDa, another possible site which explains the upper band is R186–L187 (Fig. 2C), since this site consists of arginines at the P1 and the P2 sites, a preferred pattern for cleavage by cathepsin B [24]. Cleavage at R186–L187 would generate a fragment whose molecular weight is 22–23 kDa, in agreement with the size observed on the membranes. However, this cleavage fragment appears to be in the same range as that of the mature form of the cathepsin B enzyme, suggesting that resolving the two bands may be difficult. Also, we have no evidence whether cleavage at R186 (if it occurs) happens before, after, or at the same time as that of R199. From figure 2C, however, it appears that the two fragments are generated at the same time or within a very short time frame from each other.
While the stringency for cleavage sites is lower for cathepsin B than caspases and calpains, which engage in limited proteolysis and have more defined cleavage sites, cathepsin B does not cleave randomly, and not all proteins are susceptible to cleavage by the protease. Vancompernolle et al. have shown that cathepsin B can cleave caspases in vitro, yet the efficiency of cleavage is not identical for all caspases. Proficient cleavage was observed for procaspases 1 and 11, whereas procaspases 2, 3, 6, 7, and 14 were weaker substrates, and procaspase 12 was not a substrate at all [25]. Hence, the ability of cathepsin B to cleave SK1 places SK1 among a large, but still restricted, library of substrates.
The cleavage of SK1 by cathepsin B is very likely to destroy the enzyme activity of SK1 because the H122 cleavage site lies between the proposed ATP binding site of the protease [26] and a residue that appears to be required for sphingosine binding [27]. Moreover, an SK1 mutant lacking amino acids 344–384 has been shown to have reduced binding to calmodulin, suggesting that such a deletion significantly affects the folding of the protein and potentially its activity [26]. Therefore, cleavage at R199, which generates a larger deletion of SK1, is also likely to misfold the protein and to affect its activity as well.
Previously, we have shown that SK1, lysosomes, and cathepsin B significantly colocalize in MCF-7 cells [23]. Under basal conditions, SK1 and cathepsin B may be on different faces of the lysosome: Cathepsin B on the luminal side and SK1 on the cytosolic side, suggesting that the active site of the protease does not “see” SK1. Under stimuli where lysosomes are disrupted, cathepsin B is released into the cytosol, where the pH is less acidic, inducing a shift in the specificity of the enzyme from an exopeptidase to an endopeptidase, thus enabling it to “see” SK1 and efficiently cleave it at multiple sites, resulting in loss of the protein and a subsequent decrease in SK1 activity.
In conclusion, this work has shown SK1 to be a cleavage substrate for cathepsin B and has identified protease sensitive sites in the enzyme. As such, SK1 is the first sphingolipid metabolizing enzyme shown to be directly cleaved by a protease in general, and a cysteine protease in particular.
Acknowledgments
This work was supported by the National Institutes of Health grants P01 CA097132 (to L.M.O. and Y.A.H.) and the Abney Foundation Scholarship from the Hollings Cancer Center (to T.A.T.).
References
- 1.Johnson DE. Noncaspase proteases in apoptosis. Leukemia. 2000;14:1695–703. doi: 10.1038/sj.leu.2401879. [DOI] [PubMed] [Google Scholar]
- 2.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. Embo J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polgar L, Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J Biol Chem. 1987;262:14448–14453. [PubMed] [Google Scholar]
- 4.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 5.Kominami E, Ueno T, Muno D, Katunuma N. The selective role of cathepsins B and D in the lysosomal degradation of endogenous and exogenous proteins. FEBS Lett. 1991;287:189–192. doi: 10.1016/0014-5793(91)80048-8. [DOI] [PubMed] [Google Scholar]
- 6.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 7.Roshy S, Sloane BF, Moin K. Pericellular cathepsin B and malignant progression. Cancer Metastasis Rev. 2003;22:271–286. doi: 10.1023/a:1023007717757. [DOI] [PubMed] [Google Scholar]
- 8.Guo M, Mathieu PA, Linebaugh B, Sloane BF, Reiners JJ., Jr Phorbol ester activation of a proteolytic cascade capable of activating latent transforming growth factor-betaL a process initiated by the exocytosis of cathepsin B. J Biol Chem. 2002;277:14829–14837. doi: 10.1074/jbc.M108180200. [DOI] [PubMed] [Google Scholar]
- 9.Koblinski JE, Dosescu J, Sameni M, Moin K, Clark K, Sloane BF. Interaction of human breast fibroblasts with collagen I increases secretion of procathepsin B. J Biol Chem. 2002;277:32220–32227. doi: 10.1074/jbc.M204708200. [DOI] [PubMed] [Google Scholar]
- 10.Sinha AA, Jamuar MP, Wilson MJ, Rozhin J, Sloane BF. Plasma membrane association of cathepsin B in human prostate cancer: biochemical and immunogold electron microscopic analysis. Prostate. 2001;49:172–184. doi: 10.1002/pros.1132. [DOI] [PubMed] [Google Scholar]
- 11.Sameni M, Elliott E, Ziegler G, Fortgens PH, Dennison C, Sloane BF. Cathepsin B and D are Localized at the Surface of Human Breast Cancer Cells. Pathol Oncol Res. 1995;1:43–53. doi: 10.1007/BF02893583. [DOI] [PubMed] [Google Scholar]
- 12.Muntener K, Zwicky R, Csucs G, Rohrer J, Baici A. Exon skipping of cathepsin B: mitochondrial targeting of a lysosomal peptidase provokes cell death. J Biol Chem. 2004;279:41012–41017. doi: 10.1074/jbc.M405333200. [DOI] [PubMed] [Google Scholar]
- 13.Mehtani S, Gong Q, Panella J, Subbiah S, Peffley DM, Frankfater A. In vivo expression of an alternatively spliced human tumor message that encodes a truncated form of cathepsin B. Subcellular distribution of the truncated enzyme in COS cells. J Biol Chem. 1998;273:13236–13244. doi: 10.1074/jbc.273.21.13236. [DOI] [PubMed] [Google Scholar]
- 14.Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23:2746–2756. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- 15.Stoka V, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 16.Cirman T, Oresic K, Mazovec GD, Turk V, Reed JC, Myers RM, Salvesen GS, Turk B. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J Biol Chem. 2004;279:3578–3587. doi: 10.1074/jbc.M308347200. [DOI] [PubMed] [Google Scholar]
- 17.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- 20.Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J Biol Chem. 2005;280:17196–17202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- 21.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 22.Olivera A, Barlow KD, Spiegel S. Assaying sphingosine kinase activity. Methods Enzymol. 2000;311:215–223. doi: 10.1016/s0076-6879(00)11084-5. [DOI] [PubMed] [Google Scholar]
- 23.Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM. TNF induces the loss of sphingosine kinase-1 by a cathepsin B dependent mechanism. J Biol Chem. 2005;280:17196–17202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- 24.Turk D, Guncar G, Podobnik M, Turk B. Revised definition of substrate binding sites of papain-like cysteine proteases. Biol Chem. 1998;379:137–147. doi: 10.1515/bchm.1998.379.2.137. [DOI] [PubMed] [Google Scholar]
- 25.Vancompernolle K, et al. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett. 1998;438:150–158. doi: 10.1016/s0014-5793(98)01275-7. [DOI] [PubMed] [Google Scholar]
- 26.Pitson SM, et al. The nucleotide-binding site of human sphingosine kinase 1. J Biol Chem. 2002;277:49545–49553. doi: 10.1074/jbc.M206687200. [DOI] [PubMed] [Google Scholar]
- 27.Yokota S, Taniguchi Y, Kihara A, Mitsutake S, Igarashi Y. Asp177 in C4 domain of mouse sphingosine kinase 1a is important for the sphingosine recognition. FEBS Lett. 2004;578:106–110. doi: 10.1016/j.febslet.2004.10.081. [DOI] [PubMed] [Google Scholar]


