Abstract
Development of the male urogenital tract in mammals is mediated by testicular androgens. It has been tacitly assumed that testosterone acts through its intracellular metabolite dihydrotestosterone (DHT) to mediate this process, but levels of these androgens are not sexually dimorphic in plasma at the time of prostate development. Here we show that the 3α-reduced derivative of DHT, 5α-androstane-3α,17β-diol (5α-adiol), is formed in testes of tammar wallaby pouch young and is higher in male than in female plasma in this species during early sexual differentiation. Administration of 5α-adiol caused formation of prostatic buds in female wallaby pouch young, and in tissue minces of urogenital sinus and urogenital tubercle radioactive 5α-adiol was converted to DHT, suggesting that circulating 5α-adiol acts through DHT in target tissues. We conclude that circulating 5α-adiol is a key hormone in male development.
In all mammalian species testicular androgens control three aspects of male phenotypic development—conversion of the wolffian ducts into the epididymis, vas deferens, and seminal vesicles; formation of the male urethra and prostate; and formation of the phallus (1). Studies in animals and humans with mutations in the genes that encode the androgen receptor or steroid 5α-reductase 2 indicate that testosterone virilizes the wolffian ducts and that 5α-reduced androgens are essential for formation of the prostate and phallus (1). Because virilization occurs during early fetal development in eutherian mammals, it has not been possible to measure androgens in the circulation when virilization begins.
Marsupial mammals offer an advantage for studying male phenotypic development, which takes place after birth when the young are easily accessible in the pouch and over a longer period than in the eutherian, so that it is possible to study the various aspects of phenotypic development individually (2). Virilization of the urogenital tract in the male marsupial pouch young is similar to that in eutherian fetuses, except that formation of the scrotum in marsupials is androgen-independent (2–6). This process has been examined in detail in one marsupial species, the tammar wallaby, Macropus eugenii, in which formation of the prostate begins in the urogenital sinus at around day 20 postpartum (5), whereas virilization of the penis commences around day 100 (6). Testosterone concentrations in the testes increase before prostate formation begins (7), and prostate development can be prevented by administration of the steroid 5α-reductase inhibitor finasteride (8), which blocks the formation of dihydrotestosterone (DHT), or of the androgen receptor inhibitor flutamide (9), which prevents the actions of both testosterone and DHT. Administration of large amounts of testosterone (5) or transplantation of testes to female pouch young (10) causes prostate development, whereas castration of 10-day-old male pouch young prevents formation of the prostate and penis (10).
Despite the wealth of evidence for a crucial role of 5α-reduced androgens in virilization, the circulating levels of testosterone and DHT are not sexually dimorphic at the time of prostatic and phallic development in either the tammar wallaby (11) or the gray short-tailed opossum, Monodelphis domestica (12, 13). These findings suggest that some other androgen is involved in virilization of the male marsupial. To identify the unknown agent we decided to reinvestigate testicular androgen production and metabolism in tammar wallaby pouch young at the time of prostate development between days 20 and 40 of pouch life.
Materials and Methods
Animals.
Tammar wallabies from Kangaroo Island, South Australia, were maintained in open grassy yards. These animals are seasonal breeders. During the breeding season, adult females were checked daily for births. When the day of birth was uncertain, the age of pouch young was assessed by head length (5, 7). Blood was drawn from the neck veins of 81 day-20 to -40 pouch young under hypothermic anesthesia into heparinized capillary pipettes, centrifuged, pooled, and frozen.
In Vitro Studies of Androgen Metabolism.
Testes of five male pouch young 22–36 days old (average 27 days) were pooled, and triplicate incubations were made by using three or four gonads from this pool. Gonads were added to 0.05 ml of Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY) containing 0.1 μM [1,2,6,7-3H]testosterone (3.37 TBq/mmol; Amersham Pharmacia), gassed with 95% oxygen/5% carbon dioxide, and incubated with shaking at 37°C for 2 h. Ovaries of five female pouch young aged 21–40 days (average 31 days) were incubated similarly. The reactions were stopped by the addition of 0.5 ml of acetone, and the samples were dried under air at room temperature. The residues were dissolved in 0.1 ml of chloroform/methanol (2:1), and 10-μl aliquots were spotted on 20 × 20 cm TLC plastic sheets coated with silica gel 60 (Merck, Darmstadt, Germany) together with 10 μg each of carrier steroids [testosterone, DHT, 5α-androstane-3α,17β-diol (5α-adiol), and 5α-androstane-3β,17β-diol]. The plate was developed in dichloromethane/ethyl acetate/methanol (85:15:3, vol/vol), the steroids were visualized by spraying with water, and each lane was cut into 10 fractions and assayed for radioactivity in a liquid scintillation counter.
Urogenital tracts were dissected from seven male pouch young aged 24–37 days (average 33 days), and minces of testes, urogenital tubercles, urogenital sinus, and scrotum (3.1–14.7 mg in weight) were added to 0.1 ml Dulbecco's modified Eagle's medium containing 0.1 μM [1,2-3H]testosterone (1.48 TBq/mmol), 0.1 μM [1,2-3H]DHT (1.67 TBq/mmol), or 0.1 μM 5α-[9,11-3H]adiol (2.0 TBq/mmol) (all from NEN). Duplicate samples were gassed with 95% oxygen/5% carbon dioxide and incubated at 37oC with shaking for 1 h. The reactions were stopped by acetone and the products were separated and assayed as above. Another experiment with a pool of tissues from four male pouch young 26–27 days of age but with a 2-h incubation produced essentially identical results (data not shown).
Gas Chromatography/Mass Spectrometry (GC/MS).
Androgens were measured by using selected-ion-monitoring GC/MS with deuterium-labeled androstenedione, testosterone, DHT, and 5α-adiol as internal standards (14). For the plasma assays, an internal standard mixture was added to each aliquot, and steroids were extracted twice with isooctane/methylene chloride (2:1, vol/vol). The extracts were dried and reconstituted in 5 μl of methanol and 4 ml of water, and the steroids were again extracted by using C18 Sep-Pak cartridges. For the gonadal assays, testes were weighed, homogenized, and sonicated in acetone/ethanol (1:1, vol/vol). The supernatant was aspirated, and the extraction of the pellet was repeated. The steroids in the combined supernatants were purified on 0.5-g Sephadex LH-20 columns by using the solvent system cyclohexane/ethanol (4:1, vol/vol) as described (15). Pentafluoropropionyl derivatives of the steroids in the plasma and tissue extracts were prepared and subjected to GC/MS using an HP 5971 mass selective detector, interfaced with a Hewlett-Packard 5890 gas chromatograph housing a 15-m DB-1 column. The system was programmed from 240 to 300°C. Appropriate selected ions were monitored for analytes and internal standards, and measurement of the ratios between the two provided the steroid concentrations.
The measurements were made in testes from two tammar pouch young aged 24 and 33 days and in pools of male and female tammar pouch young plasma. The A pools were collected in 1998 from 10 male pouch young aged 18–36 days, 14 female pouch young aged 18–36 days, and three adult males. The B pools were collected in 1998 from seven male pouch young aged 20–44 days and 10 female pouch young aged 20–37 days. The C pools combined all male or female plasma samples from day-21 to -40 pouch young remaining from a previous study (11).
Administration of 5α-Adiol to Female Pouch Young.
Mothers with day 19 female pouch young were randomly assigned to two treatment regimens and maintained in holding pens for 26 days. One group of six female pouch young were given 8 mg⋅kg−1⋅day−1 of 5α-adiol (Steraloids, Newport, RI) dissolved in peanut oil, and, as a control, six female pouch young were given 8 mg⋅kg−1⋅day−1 of 5β-androstane-3α,17β-diol (5β-adiol) (Steraloids) dissolved in peanut oil. The treatments were administered orally each day for 10 days through a polyethylene tube (external diameter 0.90 mm) (5). On day 45, the pouch young were killed under hypothermic anesthesia and the urogenital tracts dissected, fixed in neutral buffered formalin, and serially sectioned.
Results
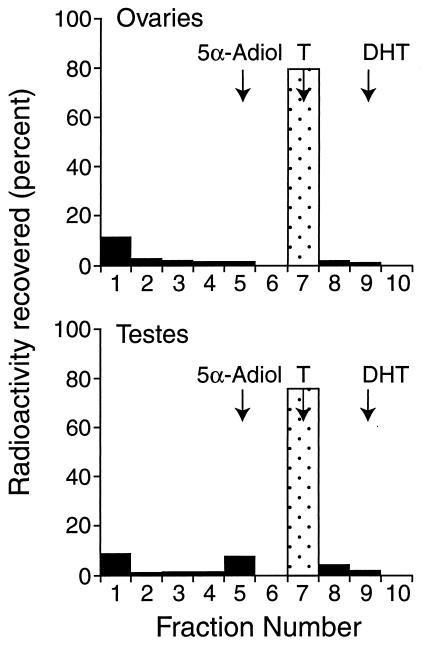
When minces of testes were incubated with tritiated testosterone in triplicate (15), 7.9%, 8.0%, and 9.4% of the radioactivity was recovered in an area corresponding to 5α-adiol, whereas ovaries converted only 0.9%, 0%, and 1.1% of the substrate to a metabolite that migrated similarly (Fig. 1). The identity of 5α-adiol in testes was confirmed by GC/MS analysis (Table 1). The concentrations of 5α-adiol and DHT in the testes of a day 24 and a day 33 pouch young averaged 64 and 62 ng⋅mg−1 wet weight, respectively, twice the average level of testosterone (34 ng⋅mg−1 wet weight). The fact that the levels of 5α-reduced androgens in testes were higher than predicted from the incubation studies indicates that 5α-reduced steroids are major products of the pouch young testes and raises the possibility that these steroids may be formed directly from progesterone as well as from testosterone, as is known to occur in the immature rat testis (16).
Figure 1.
TLC of the metabolites after the incubation of tissue fragments of testes or ovaries with tritiated testosterone. The positions of the standards on the plate are indicated by arrows. The data from one of three experiments are shown. Fraction 1 represents the origin—the metabolite at the origin in both the ovarian and testicular samples was not identified. T, testosterone.
Table 1.
GC/MS analyses of androgens in testes from tammar pouch young
| Age, days | Conc., ng⋅mg−1 wet weight of
tissue
|
||
|---|---|---|---|
| Testosterone | DHT | 5α-Adiol | |
| 24 | 31 | 62 | 49 |
| 33 | 36 | 62 | 78 |
Plasma concentrations of 5α-adiol were also assessed by GC/MS (Table 2), and the levels in day 20–40 tammar pouch young were 3 times higher in male plasma pools (averaging 1.9 ng⋅ml−1) than in female pools (averaging 0.6 ng⋅ml−1). In contrast, no consistent sexual dimorphism has been demonstrated in the levels of plasma testosterone or DHT at these ages (11). Concentrations of 5α-adiol were also higher in the pouch young than in a pool of adult male tammar plasma (0.6 ng⋅ml−1) in which the levels were similar to those in adult human males (17, 18). We know of no other report that any circulating androgen has been shown to be sexually dimorphic in a mammal at the time of commencement of prostatic development.
Table 2.
GC/MS analysis of 5α-adiol in plasma pools from day 20–40 tammar pouch young and adult male tammars
| Pool | Conc.,
ng⋅ml−1 of plasma
|
||
|---|---|---|---|
| Male pouch young | Female pouch young | Adult male | |
| A | 3.1 | 0.8 | 0.6 |
| B | 1.2 | 0.6 | — |
| C | 1.5 | 0.5 | — |
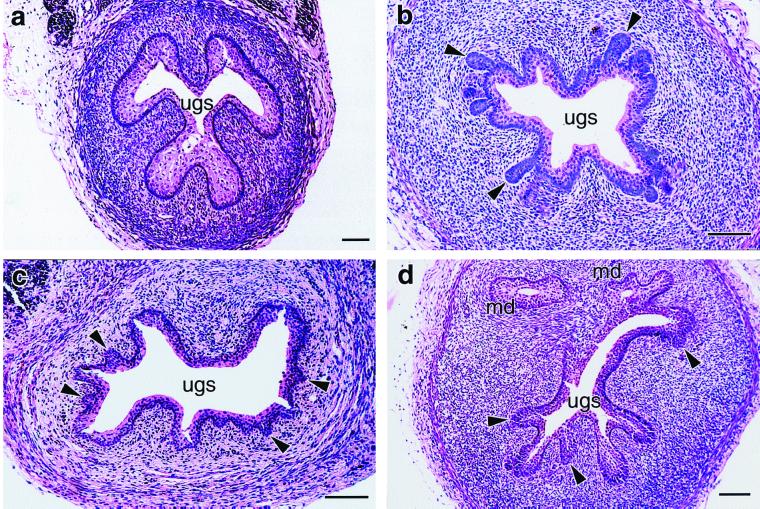
5α-Adiol is a potent pharmacological androgen. Exogenous 5α-adiol is more effective than DHT in promotion of virilization of the urogenital tract of the fetal rat (19) and more potent than DHT or testosterone in inducing prostatic hyperplasia in the castrated dog (20). 5α-Adiol also induces virilization of the urogenital sinus of female tammar pouch young (Fig. 2). In six 45-day-old female pouch young given 5α-adiol, the number of prostatic buds in 10 consecutive histological sections (11.3 ± 2.30) was not significantly different than in serial sections from five 45-day-old control males (18.0 ± 6.11, P = 0.24). No buds were observed in any of the control females given the inactive isomer 5β-adiol.
Figure 2.
Representative sections of urogenital sinus of day 45 pouch young. (a) Urogenital sinus of a control female treated with 5β-adiol contains no prostatic buds. (b) Normal male urogenital sinus with numerous prostatic buds surrounding the urogenital sinus (arrows). (c) Urogenital sinus from a female treated with 5α-adiol contains prostatic buds (arrows) similar to those of males. (d) Caudal section of urogenital sinus from female treated with 5α-adiol shows extensive branching of the urogenital sinus, with numerous large prostatic buds (arrows) not seen in males at this age. ugs, urogenital sinus; md, mullerian duct. Bar represents 0.1 mm.
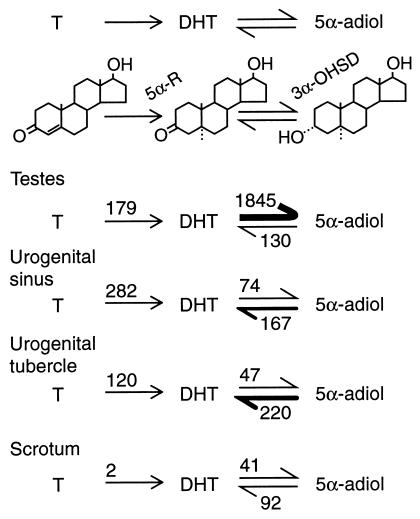
Multiple forms of the 3α-hydroxysteroid dehydrogenase enzyme exist, some of which oxidize and some of which reduce the oxygen at carbon 3 of 5α-reduced steroids (21). To examine 5α-adiol metabolism in various tammar pouch young tissues, minces of testes and urogenital tracts were incubated in vitro with radioactive testosterone, DHT, or 5α-adiol, and the metabolites were separated by TLC and assayed by scintillation spectroscopy (Fig. 3). In testes, the rate of conversion of DHT to 5α-adiol was more than 10 times the rate of either the 5α-reduction of testosterone or of the oxidation of 5α-adiol to DHT. However, in the urogenital sinus and urogenital tubercle, the rate of 5α-reduction of testosterone and the rate of oxidation of 5α-adiol to DHT exceeded the rate of conversion of DHT to 5α-adiol (Fig. 3). Thus, 5α-adiol was the predominant metabolite of testosterone in testes, whereas DHT was the principal androgen in the urogenital sinus and urogenital tubercle.
Figure 3.
Steroid hormone metabolism in minces of urogenital tissues of the tammar pouch young. Values are expressed as fmol⋅mg−1 wet weight tissue per h. T, testosterone; 5α-R, steroid-5α-reductase; 3α-OHSD, 3α-hydroxysteroid dehydrogenase.
Discussion
Although a variety of physiological functions have been postulated for 5α-adiol, such as inhibition of pituitary gonadotrophs before puberty in the rat (22) and control of the onset of parturition in the mouse (23), the present findings constitute clear evidence for a role as a crucial androgen in sexual differentiation. Four mechanisms of action have been considered for 5α-adiol. First, it could work via a typical nuclear steroid hormone receptor. However, 5α-adiol binds to the androgen receptor with a much lower affinity than DHT (21), and no evidence has been reported to date for a specific 5α-adiol receptor. Second, at the cell surface in some androgen target tissues 5α-adiol acts in combination with sex hormone-binding globulin to enhance cAMP production and to activate secondarily the androgen receptor (24). Because the tammar wallaby does not have a circulating sex hormone-binding globulin (25), it is unlikely that 5α-adiol acts via a cell surface receptor in this species. Third, in the brain 5α-adiol inhibits the γ-aminobutyric acid receptor, a system that may or may not function in other tissues (26). Finally, 5α-adiol may act by back conversion to DHT because a large fraction of radioactive 5α-adiol is recovered in the form of DHT in putative sites of androgen action (27, 28) and because DHT is formed from both testosterone and 5α-adiol in the prostates of the dog (29) and rat (27). The metabolic studies reported here indicate that the urogenital sinus and urogenital tubercle of the developing tammar convert 5α-adiol to DHT, which then can act via the androgen receptor (1). The concept that 5α-adiol acts via back conversion to DHT is strengthened by the fact that the androgen receptor antagonist flutamide inhibits prostate formation in the tammar (9). Why the active metabolite DHT would be formed by such an indirect mechanism is unknown.
In summary, the present studies demonstrate that 5α-adiol is formed in the testes and is the circulating androgen responsible for prostatic development in tammar wallaby pouch young. It remains to be seen whether it plays a similar role in other tissues of the male tammar or in other mammals, although it is known to be present in the early human fetal testes (30) and in the immature rat testis (16).
Acknowledgments
We thank Roger V. Short, Joseph L. Goldstein, and David W. Russell for helpful comments on the manuscript. This work was supported by grants from the National Health and Medical Research Council (Australia), The University of Melbourne, and the University of Texas Southwestern Medical Center. C.H.L.S. is supported by the endowment of the Children's Hospital Oakland Research Institute. All experiments followed guidelines of the National Health and Medical Research Council of Australia and were approved by the University of Melbourne Animal Ethics Committees.
Abbreviations
- DHT
dihydrotestosterone
- 5α-adiol
5α-androstane-3α,17β-diol
- 5β-adiol
5β-androstane-3α,17β-diol
- GC/MS
gas chromatography/mass spectrometry
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220412297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220412297
References
- 1.Wilson J D, George F W. In: The Physiology of Reproduction. 2nd Ed. Knobil E, Neill J, editors. Raven Press; 1994. pp. 3–28. [Google Scholar]
- 2.Renfree M B, Harry J L, Shaw G. Philos Trans R Soc London B. 1995;350:243–258. doi: 10.1098/rstb.1995.0158. [DOI] [PubMed] [Google Scholar]
- 3.O W-S, Short R V, Renfree M B, Shaw G. Nature (London) 1988;331:716–717. doi: 10.1038/331716a0. [DOI] [PubMed] [Google Scholar]
- 4.Renfree M B, Short R V. Philos Trans R Soc London B. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- 5.Shaw G, Renfree M B, Short R V, O W-S. Development (Cambridge, UK) 1988;104:689–701. doi: 10.1242/dev.104.4.689. [DOI] [PubMed] [Google Scholar]
- 6.Butler C M, Shaw G, Renfree M B. Anat Embryol. 1999;199:451–457. doi: 10.1007/s004290050243. [DOI] [PubMed] [Google Scholar]
- 7.Renfree M B, Wilson J D, Short R V, Shaw G, George F W. Biol Reprod. 1992;47:644–647. doi: 10.1095/biolreprod47.4.644. [DOI] [PubMed] [Google Scholar]
- 8.Ryhorchuk A R, Shaw G, Butler C M, Renfree M B. J Androl. 1997;181:123–130. [PubMed] [Google Scholar]
- 9.Lucas J C, Renfree M B, Shaw G, Butler C M. J Reprod Fertil. 1997;109:205–212. doi: 10.1530/jrf.0.1090205. [DOI] [PubMed] [Google Scholar]
- 10.Tyndale-Biscoe C H, Hinds L A. Reprod Fertil Dev. 1989;1:243–254. doi: 10.1071/rd9890243. [DOI] [PubMed] [Google Scholar]
- 11.Wilson J D, George F W, Shaw G, Renfree M B. Biol Reprod. 1999;61:471–475. doi: 10.1095/biolreprod61.2.471. [DOI] [PubMed] [Google Scholar]
- 12.Fadem B H, Harder J D. Biol Reprod. 1992;46:105–108. doi: 10.1095/biolreprod46.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Xie Q, Mackay S, Ullmann S L, Gilmore D P, Payne A P, Gray C. Biol Reprod. 1998;58:664–669. doi: 10.1095/biolreprod58.3.664. [DOI] [PubMed] [Google Scholar]
- 14.Wudy S A, Wachter U A, Homoki J, Teller W M, Shackleton C H L. Steroids. 1992;57:319–324. doi: 10.1016/0039-128x(92)90050-j. [DOI] [PubMed] [Google Scholar]
- 15.Shackleton C H L, Phillips A, Chang T, Li Y. Steroids. 1997;62:379–387. doi: 10.1016/s0039-128x(96)00253-x. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein B, Borut A, Cohen S. Biochim Biophys Acta. 1987;924:1–6. doi: 10.1016/0304-4165(87)90063-8. [DOI] [PubMed] [Google Scholar]
- 17.Jacolot F, Adeline J, Berthou F, Floch H H. J Steroid Biochem. 1985;22:533–557. doi: 10.1016/0022-4731(85)90174-8. [DOI] [PubMed] [Google Scholar]
- 18.Salerno R, Moneti G, Forti G, Magini A, Natali A, Saltutti C, Di Cello V, Costantini A, Serio M. J Andrology. 1988;9:234–240. doi: 10.1002/j.1939-4640.1988.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 19.Schultz F M, Wilson J D. Endocrinology. 1974;94:979–986. doi: 10.1210/endo-94-4-979. [DOI] [PubMed] [Google Scholar]
- 20.Moore R J, Gazak J M, Quebbeman J F, Wilson J D. J Clin Invest. 1979;64:1003–1010. doi: 10.1172/JCI109536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penning T M. Endocr Rev. 1997;18:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 22.Ruf K B. J Steroid Biochem. 1983;19:887–890. doi: 10.1016/0022-4731(83)90029-8. [DOI] [PubMed] [Google Scholar]
- 23.Mahendroo M S, Cala K M, Russell D W. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 24.Ding V D, Moller D E, Feeney W P, Didolkar V, Nakhla A M, Rhodes L, Rosner W, Smith R G. Endocrinology. 1998;139:213–218. doi: 10.1210/endo.139.1.5681. [DOI] [PubMed] [Google Scholar]
- 25.Sernia C, Bradley A J, McDonald I R. Gen Comp Endocrinol. 1979;38:496–503. doi: 10.1016/0016-6480(79)90159-x. [DOI] [PubMed] [Google Scholar]
- 26.Frye C A, Van Keuren K R, Ersking M S. Behav Brain Res. 1996;79:109–118. doi: 10.1016/0166-4328(96)00004-6. [DOI] [PubMed] [Google Scholar]
- 27.Bruchovsky N. Endocrinology. 1971;89:1212–1222. doi: 10.1210/endo-89-5-1212. [DOI] [PubMed] [Google Scholar]
- 28.Pilven A, Thieulant M L, Ducouret B, Samperez S, Jouan P. Steroids. 1976;28:349–358. doi: 10.1016/0039-128x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- 29.Jacobi G H, Moore R J, Wilson J D. Endocrinology. 1978;102:1748–1758. doi: 10.1210/endo-102-6-1748. [DOI] [PubMed] [Google Scholar]
- 30.George F W, Carr B R, Noble J F, Wilson J D. J Clin Endocrinol Metab. 1987;64:628–630. doi: 10.1210/jcem-64-3-628. [DOI] [PubMed] [Google Scholar]