Abstract
It is well established that motor neurons with large axon caliber are selectively affected in amyotrophic lateral sclerosis (ALS). To investigate whether high neurofilament (NF) content and large axonal caliber are factors that predispose motor neurons to selective degeneration in ALS, we generated mice expressing a mutant form of superoxide dismutase 1 (SOD1G37R) linked to familial ALS in a context of one allele for each NF gene being disrupted. A ≈40% decrease of NF protein content detected in triple heterozygous knockout mice shifted the calibers of large axons in L5 ventral root from 5–9 μm to 1–5 μm, altering neither the normal subunit stoichiometry and morphological distribution of NFs nor levels of other cytoskeletal proteins. This considerable reduction in NF burden and caliber of axons did not extend the life span of SOD1G37R mice nor did it alleviate the loss of motor axons. Moreover, increasing the density of NFs in axons by overexpressing a NF-L transgene did not accelerate disease in SOD1G37R mice. These results do not support the current view that high NF content and large caliber of axons may account for the selective vulnerability of motor neurons in ALS caused by mutant SOD1.
Keywords: amyotrophic lateral sclerosis, Cu/Zn superoxide dismutase, neurofilaments, transgenic mice, knockout mice
Amyotrophic lateral sclerosis (ALS) is a progressive adult-onset neurodegenerative disorder that affects primarily large motor neurons in the brain and spinal cord. Selective degeneration of these motor neurons leads to atrophy of skeletal muscles and ultimately to paralysis and death within 1 to 5 years (1). Approximately 10% of ALS cases are inherited in an autosomal dominant manner (1), and ≈20% of these familial ALS patients have missense mutations in the gene coding for the superoxide dismutase 1 (SOD1), a metalloenzyme catalyzing the conversion of superoxide anions into hydrogen peroxide (1).
The selective vulnerability of motor neurons in ALS is not well understood. Many hypotheses have been suggested to account for such vulnerability, including decreased levels of calcium-binding proteins calbindin-D28K and parvalbumin in motor neurons (2, 3), the decreased amount of collagen in the posterior half of lateral funiculus and in the anterior horn (4), expression of atypical calcium-permeable AMPA receptors in human motor neurons (5), differential distribution of metabotropic glutamate receptor in somatic motor neurons (6), and poor antioxidant defenses in motor neurons (1, 7). In addition, the most prevailing hypothesis is that a high content in neurofilament (NF) proteins and a large caliber of axons may contribute to the selective vulnerability of motor neurons in ALS (1, 8–10).
NF proteins are composed of three subunits: NF-L (61 kDa), NF-M (90 kDa), and NF-H (110 kDa). They belong to the type IV intermediate filaments (IFs) (11, 12). NFs are obligate heteropolymers requiring NF-L together with either NF-M or NF-H for polymer formation (11–14). The three subunits share with other members of the IF family a central domain of approximately 310 amino acids, which is involved in the formation of coiled-coil dimers (11, 12). After their synthesis in the perikaryon, the three NF subunits are assembled in the cytoplasm and are moved down the axon with the slow axonal transport component (11, 12). A Japanese quail mutant and mice knockout for the NF-L gene provided definite proof that NFs are a major determinant in the radial growth of large myelinated axons (15, 16).
Three independent studies reported the selective degeneration of motor neurons with large axonal diameter (α motor neurons) in ALS patients, whereas motor neurons with small axonal caliber (γ motor neurons) were spared (8–10). Because NFs are a major determinant of axonal caliber, it has been suggested that a high axonal content in NFs could predispose to degeneration in ALS. This view was supported by the detection of abnormal NF accumulations in spinal motor of ALS patients (17, 18) and by the finding that overexpression of some NF transgenes could provoke motor neuron disease (19, 20). Compelling evidence for NF involvement in ALS was also provided by the discovery of NF-H mutations associated with a small number of ALS cases (21–23).
Transgenic mice overexpressing mutant SOD1 develop ALS-like phenotypes through a gain of unidentified deleterious activities (24–30). To test the contribution of NFs to motor neuron disease caused by mutant SOD1, mice expressing mutant SOD1 were mated with mice having altered NF protein levels. Remarkably, the life span of mice expressing mutant SOD1 was significantly delayed as a consequence of NF-L disruption (31). However, it has remained unresolved whether the slowing down of disease in this experiment was the consequence of a depletion of deleterious NFs in motor axons or of an accumulation of NF proteins in the perikaryon of motor neurons. The overexpression of human NF-H proteins, which raises perikaryal NF levels and lowers axonal levels, conferred remarkable protection in SOD1G37R mice (32).
To clarify the contribution of axonal caliber to motor neuron disease, we generated SOD1G37R mice in a context of one disrupted allele for each NF gene. Triple heterozygous knockout (TH) mice exhibited a 40% decrease in levels of each NF protein with corresponding reduction in caliber of large motor axons in the L5 ventral root. This approach allowed us to reduce the caliber of axons without altering the normal subunit stoichiometry and morphological distribution of NFs or levels of other cytoskeletal proteins. This considerable decrease in NF content did not extend the life span of SOD1G37R mice, and motor axons with reduced calibers remained equally vulnerable to degeneration. These results do not support the current view that the caliber of axons is a factor contributing to the selective vulnerability of motor neurons in ALS.
Materials and Methods
Generation of SOD1G37R Mice with Altered Levels of NF Proteins.
NF-L, NF-M, NF-H knockout, transgenic human NF-L, and transgenic SOD1G37R mice were generated as described previously (13, 16, 26, 33, 34). Mice double knockout for NF-M and NF-H genes (C57BL6-enriched strain) were bred with SOD1G37R+/−;NF-L null mice (C57BL6-enriched strain). Half of the progeny had the genotype NF-L+/−, NF-M+/−, NF-H+/− (TH), and the other half had the SOD1G37R;TH genotype. The SOD1G37R+/− mice in C57BL6 background were used as controls for the survival curve. Transgenic hNF-L+/+ mice (C57BL6 inbred) were bred with SOD1G37R+/− mice (C57BL6 inbred). Half of the offspring had the hNF-L+/− genotype, and the other half had the SOD1G37R+/−;hNF-L+/− genotype. The SOD1G37R+/− mice in C57BL6 background were used as controls for the survival curve. The life span of our SOD1G37R mouse line 29 in a pure C57BL6 background is about ≈52 weeks, similar to the life span of inbred C57BL6 SOD1G37R mice (line 29) derived by Eyer et al. (35). The use of animals and all surgical procedures described in this article were carried out according to The Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care (www.ccac.ca).
RNA Analysis.
Total RNA from spinal cord was isolated by using the Trizol reagent (GIBCO/BRL). RNA (10 μg) was fractionated on a 1% agarose formaldehyde gel before blotting on a Hybond N+ membrane (Amersham). Blots were hybridized with specific 32P-labeled DNA probes for actin and each NF gene. All quantitation was done by densitometry by using gel-pro analyzer (Media Cybernetics, Silver Spring, MD) and image quant (Molecular Dynamics) softwares. Results were normalized with levels of actin mRNA.
SDS/PAGE and Western Blot Analysis.
Mice were killed by intraperitoneal injection of chloral hydrate. Tissue samples were frozen in liquid nitrogen and stored at −80°C. Total protein extracts from spinal cord were obtained by homogenization of tissue samples in SDS-urea β-mercaptoethanol (0.5% SDS/8 M urea in 7.4 phosphate buffer) with a mixture of protease inhibitors (PMSF, leupeptin, pepstatin, apoprotinin). The supernatant was collected after centrifugation at 10,000 × g for 20 min. The protein concentration was estimated by the Bradford procedure (Bio-Rad). Proteins (5 μg) were fractionated on 7.5% SDS/PAGE and blotted on a nitrocellulose membrane for Western blot analysis. The monoclonal antibodies against NF-L (NR 4), NF-M (NN 18), NF-H (RT 97), β-tubulin, and actin (C4) were purchased from Boehringer Mannheim. The antibodies against the hypophosphorylated form of NF-H (SMI 32) are from Sternberger–Meyer, Jarrettsville, MD, whereas the antiperipherin (AB 1530) and anti-α-internexin (AB 1515) polyclonal antibodies came from Chemicon (Mississauga, Canada). The Western blot results were revealed by RENAISSANCE, a Western blot chemiluminescence kit from NEN. All quantitation was done by densitometry by using the gel-pro analyzer (Media Cybernetics) and image quant (Molecular Dynamics) softwares. The results were normalized with levels of actin.
Immunochemistry and Morphological Analysis.
Mice were killed by overdose of chloral hydrate, perfused with 0.9% NaCl, and then with fixative (3% vol/vol glutaraldehyde in PBS buffer, pH 7.4). Tissue samples were immersed in fixative overnight, rinsed in phosphate buffer, and then postfixed in 1% phosphate-buffered osmium tetroxide. After three washes with phosphate buffer, each sample was dehydrated in a graded series of ethanol and embedded in Epon (Marivac, Halifax, Canada). Thin sections of spinal cord and L5 ventral root were stained with Toluidine blue and examined under a light microscope. For immunocytochemistry studies, 50-μm spinal cord sections from glutaraldehyde-perfused mice were prepared by using a vibratome. Floating sections were rinsed in PBS and treated for 30 min with a 1% (wt/vol) sodium borohydride solution to reduce epitope masking by glutaraldehyde. The sections were then blocked for 1 h in a PBS solution containing 3% (wt/vol) BSA, 0.5% (vol/vol) Triton X-100, and 0.03% (wt/vol) hydrogen peroxide. Incubation with different antibodies was performed overnight at 4°C with agitation in a PBS solution containing 3% (wt/vol) BSA and 0.05% (wt/vol) Triton X-100. Immunohistochemistry staining was developed by using a Vector ABC kit (Vector Laboratories) and SIGMAFAST tablets (Sigma). Counting of axons and measurement of axonal diameter in the L5 ventral root were carried out with image-1 software from Universal Imaging.
Results
mRNA and Protein Levels in Mice Having One Disrupted Allele for Each NF Gene.
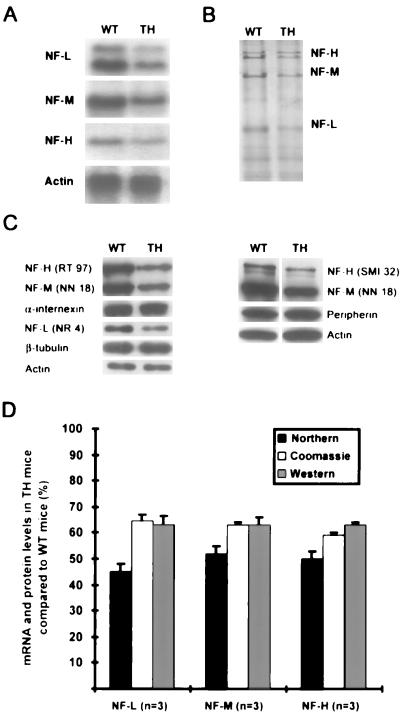
Mice double knockout for NF-M and NF-H genes were bred with SOD1G37R+/−;NF-L null mice (see Materials and Methods). Half of the progeny had the genotype NF-L+/−, NF-M+/−, NF-H+/− (TH), and the other half had the SOD1G37R;TH (SOD;TH) genotype. Mouse genotypes were established by Southern blotting of tail DNA (data not shown). Northern blot analysis showed a 50% reduction in mRNA levels for each NF subunit in the spinal cord of TH mice as compared with normal mice (Fig. 1A). Densitometry of Coomassie blue-stained SDS/PAGE demonstrated a ≈40% decrease in protein levels for each of the three NF subunits in spinal cord (Fig. 1B). The 40% decrease in the NF-H protein level in TH mice has been confirmed by Western blotting by using the RT 97 and SMI 32 antibodies, recognizing respectively the phosphorylated and hypophosphorylated forms of NF-H (Fig. 1C). It is noteworthy that no changes in phosphorylation of NF-H occur to compensate for the decreased levels of NF proteins (Fig. 1C). The 40% reduction in NF-M and NF-L protein levels has been confirmed with the NN 18 antibody and the NR 4 antibody, respectively. The levels of peripherin, α-internexin, and β-tubulin remained unchanged in TH mice (Fig. 1C). All quantitation was done by densitometry by using gel-pro imaging and image quant softwares. The results, which were normalized with levels of actin, are shown in Fig. 1D. In the brain, the mRNA and protein levels for each NF subunit also decreased by 50 and 40%, respectively (data not shown).
Figure 1.
mRNA and protein levels in spinal cord of TH mice. (A) Northern blot shows a 50% reduction in mRNA levels for each NF gene in spinal cord samples of TH mice. (B) Reduction in NF mRNA induced a 40% decrease in NF protein levels in TH mice as detected by SDS/PAGE stained with Coomassie blue. (C) This reduction in NF protein levels was confirmed by Western blotting by using antibodies for NF-L (NR 4), NF-M (NN 18), and phosphorylated and hypophosphorylated NF-H (RT 97, SMI 32). Note that levels of peripherin, α-internexin, β-tubulin, and actin in TH mice are identical to levels occurring in normal mice. (D) Histograms summarizing the mRNA and protein levels as determined by densitometry measurement and normalization with levels of actin.
Reduced Calibers of Ventral Root Axons in TH Mice.
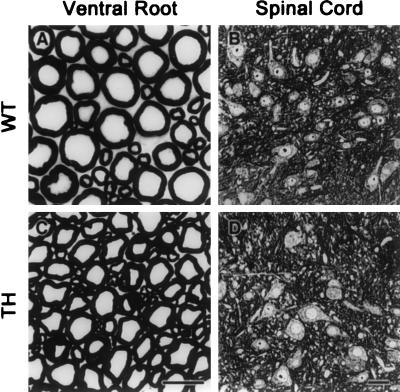
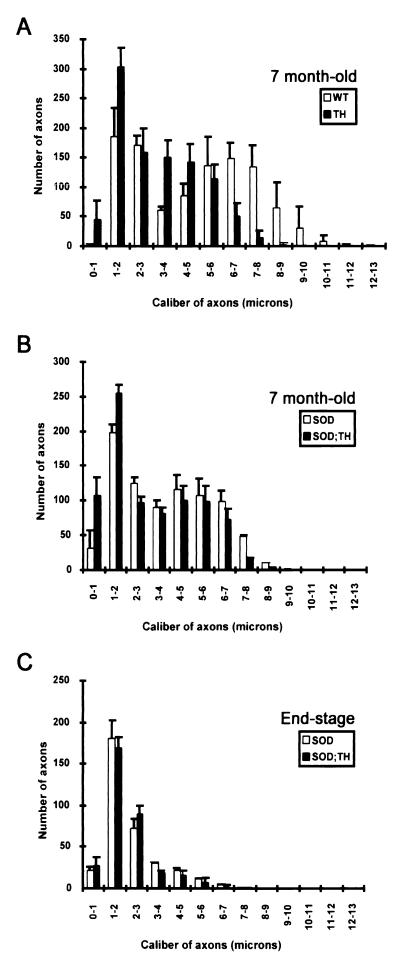
L5 ventral roots from 7-month-old mice were examined with light microscopy. As shown in Fig. 2, calibers of large motor axons in the L5 ventral root of TH mice are substantially reduced compared with those from normal mice. To quantify the changes in axonal calibers, cross-sectional areas of L5 ventral root axons were analyzed by using a morphometric software (image 1, Universal Imaging). Normal mice exhibit a bimodal distribution of axonal caliber with peaks at ≈2 μm and ≈7 μm in diameter (Fig. 3A). In contrast, no bimodal distribution is observed in TH mice, and the majority of axons are in the range of 1–5 μm in diameter. The majority of large axons occurring in the range of 5–9 μm in normal mice have been shifted to smaller sizes in the range of 1–5 μm in TH mice. There was a 20% increase (an increase of 200 axons) in the number of motor axons with 3- to 5-μm caliber and a 15% increase (an increase of 150 axons) in the number of motor axons with 1- to 2-μm caliber (Fig. 3A). Thus, the 40% decrease in levels of all three NF proteins detected in TH mice (Fig. 1) led to a corresponding reduction in the caliber of ventral root axons. No significant loss of motor axons was detected in TH mice (Table 1). The number of axons in the L5 ventral root of 7-month-old TH mice was 981 ± 90 axons (n = 5), not significantly different from the number 1,031 ± 57 axons (n = 6) measured in normal mice (Table 1).
Figure 2.
Light micrographs of L5 ventral root and spinal cord. Transverse sections of L5 ventral root and spinal cord from normal (WT) mice and mice with one allele deleted for each NF gene (TH) at 7 months old. The disruption of one allele for each NF gene reduced substantially the radial growth of motor axons (B) but did not change the morphology of spinal motor neurons (D). [Bars = (A and C) 12 μm; (B and D) 25 μm.]
Figure 3.
Caliber distribution of axons in L5 ventral roots. (A) Caliber distribution of axons in the L5 ventral root of TH (n = 6) and normal (WT) (n = 6) mice at 7 months old. The disruption of one allele for each NF gene shifted the caliber of large axons from a range of 5–9 μm to 1–5 μm. (B) Caliber distribution of axons in the ventral root of SOD1G37R (n = 4) and SOD1G37R;TH (n = 3) 7-month-old mice. Note the loss of motor axons with reduced caliber (2- to 5-μm range) in the TH mice expressing SOD1G37R. (C) Caliber distribution of ventral root axons at the end stage of disease. The SOD1G37R (n = 3) and SOD1G37R;TH (n = 3) mice exhibited similar distribution of remaining axons at the end stage of disease. Therefore, motor axons with reduced caliber remained equally vulnerable to degeneration in disease caused by mutant SOD1.
Table 1.
Axonal counts of L5 ventral root
| Genotype | Number of axons at 7 months old | Number of axons at end stage of disease |
|---|---|---|
| WT | 1,031 ± 57 (n = 6) | |
| TH | 981 ± 90 (n = 5) | |
| SOD1G37R | 824 ± 66 (n = 4) | 341 ± 18 (n = 3) |
| SOD1G37R;TH | 831 ± 91 (n = 3) | 327 ± 14 (n = 3) |
Normal Perikaryal NF Content in Spinal Motor Neurons of TH Mice.
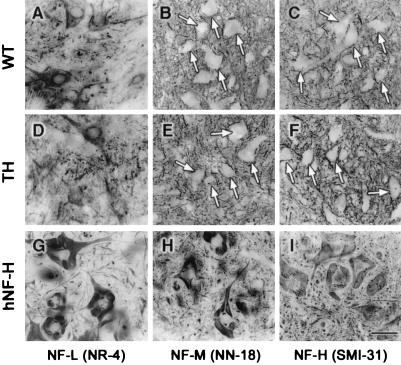
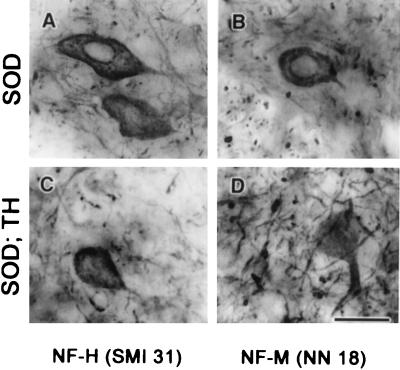
Many mouse models with altered subunit stoichiometry of NFs exhibit perikaryal accumulation of NF proteins. For instance, mice single knockout for the NF-L and NF-M genes as well as mice double knockout for NF-M and NF-H showed enhanced levels of NF proteins in the perikaryon of spinal motor neurons (13, 16, 31). Moreover, as shown in Fig. 4 G–I, transgenic mice overexpressing human NF-H alone develop large perikaryal NF accumulations containing all three NF subunits (20, 32). These results suggest a requirement for correct subunit stoichiometry to achieve efficient translocation of NF proteins from the neuronal perikaryon into the axonal compartment. With light microscopy, the spinal motor neurons of TH mice did not differ from those of normal mice in contrast to spinal motor neurons of hNF-H transgenic mice that exhibit accumulations of NFs in perikarya (Fig. 2 B and D and Fig. 7A Left). In addition, immunostaining of spinal cord sections with antibodies against NF-L (NR 4), NF-M (NN 18), and phosphorylated NF-H (SMI 31) proteins showed a similar distribution of NF proteins in samples from TH mice and normal mice (Fig. 4 A–F). No accumulation of phosphorylated NF proteins was detected in neuronal perikarya of TH mice (Fig. 4 B and C, E and F). Thus, decreasing the NF protein levels without altering the subunit stoichiometry does not affect the normal distribution and properties of NF proteins. It is noteworthy that despite the 40% decrease in NF levels, the SOD1G37R;TH mice exhibited increased immunoreactivities for NF-H (SMI 31) and NF-M (NN 18) in the perikaryon of spinal motor neurons, like mice expressing SOD1G37R in the normal NF background (Fig. 5 A–D).
Figure 4.
No perikaryal accumulation of NF proteins in TH mice. As shown in A–F, normal and TH mice exhibited similar immunohistochemical staining of the spinal cord with antibodies against NF-L (NR 4) (A and D), NF-M (NN 18) (B and E), and NF-H (SMI 31) (C and F). White arrows in B, C, E, and F point to perikaryon of motor neurons. In contrast, the spinal cord from transgenic mice overexpressing hNF-H used here as positive controls showed immunodetection of NF proteins in the perikarya of motor neurons (G–I). [Bar = (I) 50 μm.]
Figure 7.
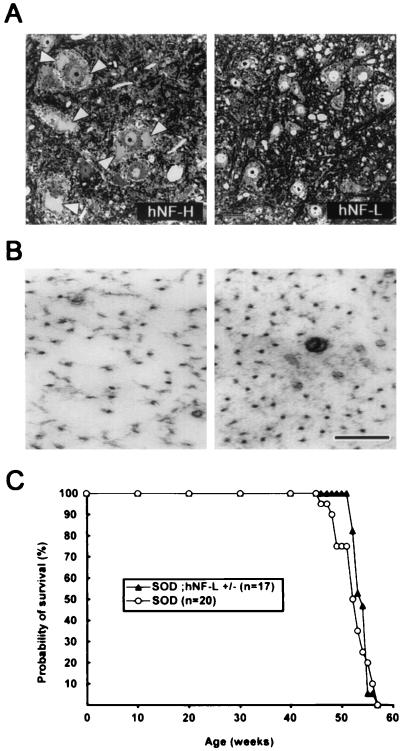
Increasing NF density in axons does not exacerbate disease caused by mutant SOD1G37R. (A) Transverse sections of spinal cord from hNF-H or hNF-L transgenic mice at 7 months old. The overexpression of hNF-H caused large accumulations of NF in neuronal perikarya (Left, white arrowheads). In contrast, hNF-L transgenic mice do not develop such NF accumulations (Right). (Bar = 25 μm.) (B) Electron micrographs revealed a ≈50% increase of axonal NF content in motor axons of 7-month-old SOD1G37R mice overexpressing hNF-L (Right) compared with axons of age-matched SOD1G37R mice (Left). (Bar = 100 nm.) (C) Survival curves of mice expressing SOD1G37R alone or together with a hNF-L transgene. The survival probability of transgenic mice is plotted as a function of their age in weeks. The overexpression of hNF-L, which increased the axonal NF content by ≈50% without altering axonal caliber, did not accelerate disease caused by SOD1G37R.
Figure 5.
Perikaryal NF immunodetection in SOD1G37R and SOD1G37R;TH mice. Both SOD1G37R and SOD1G37R;TH mice exhibited immunohistochemical staining of the spinal cord with antibodies against NF-M (NN 18) (B and D) and NF-H (SMI 31) (A and C). (Bar = 15 μm.)
Life Span of SOD1G37R Mice Unaffected by Reduction of Axonal Calibers.
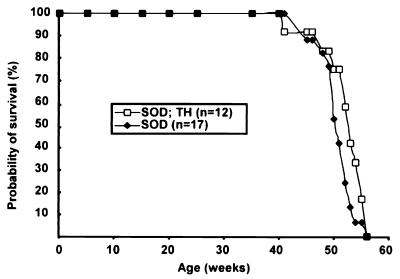
Fig. 6 shows the survival curves of SOD1G37R mice (line 29) in wild-type NF and TH backgrounds. The SOD1G37R mice (n = 17) used as control exhibited an average life span of 50.6 ± 2.8 weeks and a median of life probability of 51.0 weeks. The median of life probability is defined as the age at which the probability of survival is 50%. The SOD1G37R;TH mice (n = 12) exhibited an average life span of 51.8 ± 4.0 weeks and a median of life probability of 52.5 weeks. Table 2 summarizes the median of life probability and average life span with standard deviation for each genotype. These results demonstrate that the life span of SOD1G37R mice was unaffected by the decreased levels of NF content and reduction of calibers in TH background.
Figure 6.
Reducing axonal caliber does not extend life span of SOD1G37R mice. Transgenic mice expressing SOD1G37R in the wild-type NF background or in a context of disruption of one allele for each NF gene (TH) yielded similar survival curves. The survival probability of transgenic mice is plotted as a function of their age in weeks.
Table 2.
Life span of SOD1G37R mice with one allele disrupted for each NF gene or with hNF-L overexpression
| Genotype | Average life span, weeks | Median of life probability, weeks |
|---|---|---|
| SOD1G37R (n = 17) | 50.6 ± 2.8 | 51.0 |
| SOD1G37R;TH (n = 12) | 51.8 ± 4.0 | 52.5 |
| SOD1G37R (n = 20) | 52.5 ± 3.0 | 52.5 |
| SOD1G37R;hNF-L (n = 17) | 53.9 ± 1.4 | 54.0 |
To compare the selective vulnerability of motor axons to degeneration, we counted the number of axons and measured their caliber in L5 ventral roots of SOD1G37R and SOD1G37R;TH mice at 7 months old and at end stage of disease. At 7 months old, the SOD1G37R;TH mice (n = 3) had 831 ± 91 axons, whereas the SOD1G37R mice (n = 4) had 824 ± 66 (Table 1). Thus, at this age, there was a similar loss of motor axons in both mouse strains. As shown in Fig. 3B, there was a preferential loss of the larger caliber axons in the range of 5–9 μm from the L5 ventral root of SOD1G37R mice (n = 3) at 7 months old. In the 7-month-old SOD1G37R;TH mice (n = 3), the larger caliber axons were also those that degenerated, albeit their caliber was considerably reduced to the 1- to 5-μm range. Therefore, reducing the axonal caliber of the motor neurons at risk did not protect them against toxicity of mutant SOD1. This was further confirmed by examination of L5 ventral root axons at the end stage of disease. At end stage, the SOD1G37R;TH and SOD1G37R mice exhibited similar number of axons and caliber distribution (Fig. 3C and Table 1). The SOD1G37R;TH mice (n = 3) had 327 ± 14 axons, whereas the SOD1G37R mice (n = 3) had 341 ± 18 (Table 1). Our interpretation is that motor neurons with a large caliber of axons remain at risk from the toxicity of mutant SOD1 despite caliber reduction. Thus, those axons in SOD1G37R;TH mice did not need their high NF content and their normal axon calibers to be at risk. It is particularly striking that at end stage, the SOD1G37R;TH mice exhibited, like the SOD1G37R mice, only ≈200 axons in the range of 1- to 2-μm caliber, even though their initial number of axons in this range was much higher (≈350 axons; see Fig. 3A).
Disease Caused by SOD1G37R Mice Not Exacerbated by Higher NF Density.
To further investigate the effect of NFs in disease caused by SOD1G37R, we mated SOD1G37R mice with mice homozygous for the human NF-L transgene (hNF-L+/+), whose motor neurons do not exhibit accumulations of perikaryal NFs in contrast to hNF-H overexpressor mice (Fig. 7A, white arrowheads). When compared with SOD1G37R mice (Fig. 7B Left), the SOD1G37R;hNF-L+/− mice exhibited a ≈50% increase in the NF content in axons without changes in axonal calibers (Fig. 7B Right and ref. 36). Fig. 7C shows the survival curve of these mice. SOD1G37R mice (n = 20) exhibited an average life span of 52.5 ± 3.0 weeks and a median of life probability of 52.5 weeks, whereas the SOD1G37R mice overexpressing hNF-L (n = 17) exhibited an average life span of 53.9 ± 1.4 weeks and a median of life probability of 54.0 weeks (Table 2). Therefore, increasing the density of NFs in axons did not exacerbate disease caused by SOD1G37R.
Discussion
The results presented here indicate that high NF burden and large caliber of axons do not account for the selective vulnerability of motor neurons to toxicity of mutant SOD1 in familial ALS. Many lines of evidence led to the belief that the caliber of axons contributes to vulnerability of motor neurons to degeneration in ALS. It is well established that there is a selective loss of large myelinated axons in ventral roots from ALS patients, whereas small axons are spared (1, 8–10). One favored explanation for the selective loss of large axons was that NFs, which are more abundant in large axons, might represent targets of oxidative damage and perhaps might contribute to disease (for review, see ref. 1). In vitro studies demonstrated that the NF-L protein could be a target of tyrosine nitration arising from enhanced use of peroxynitrite by mutant SOD1 (37). This led to the possibility that nitrated NF-L could affect NF organization and provoke noxious NF accumulations in axons. The zinc-chelating property of NF-L has recently been proposed as another possible explanation for detrimental effects of a high NF content. The NF-L protein may compete with SOD1 for zinc binding (37), and zinc-deficient SOD1 was found to induce motor neuron death in culture (38). The view that axonal NFs could contribute to selective vulnerability of motor neurons in SOD1-mediated disease is compatible with the findings that absence of NF-L extends by 20% the life span of mice overexpressing either SOD1G85R (31) or SOD1G37R (unpublished results). However, these experiments were difficult to interpret because the beneficial effect of NF-L disruption could arise from an increase in perikaryal levels of NF-H and NF-M proteins rather than from a depletion of NFs in axons. For instance, overexpression of human NF-H proteins in SOD1G37R mice induced large perikaryal NF accumulations with remarkable protective effect (32).
The TH mice described here provide a mouse model with decreased NF content and reduction of axon calibers without changes in normal subunit stoichiometry and NF distribution or in levels of other cytoskeletal proteins. There are previous reports of mouse models with reduced axonal calibers, but these mice also exhibit morphological and pathological changes such as increased levels of perikaryal NF proteins, dendritic attrition, and variations in levels of other cytoskeletal proteins that complicate interpretation of results (13, 16, 19, 20, 39–44). By disrupting one allele for each NF gene, it was possible to preserve the normal subunit stoichiometry and integrity of the NF network. As a consequence, no perikaryal sequestration of NF proteins occurred in the TH mice.
Mice with TH background exhibited a substantial decrease of ≈40% in NF content and a corresponding reduction in the caliber of large axons. As shown in Fig. 3, the calibers of large myelinated axons in the L5 ventral root that occur normally in the range of 5–9 μm have been shifted to sizes in the 1- to 5-μm range. On the basis of axonal regeneration studies in mice expressing the SOD1G93A mutant, Kong et al. (45) recently suggested that there is a threshold of selective vulnerability of motor axons to degeneration at 4–5 μm in caliber. Interestingly, axotomy was found to reduce the extent of motor neuron degeneration at the end stage of disease in SOD1G93A mice (45). Because all surviving motor axons exhibited diameters smaller than 4.5 μm, this led to hypothesize an apparent threshold of vulnerability correlating with axon size (45). However, this suggestion is not supported by the results presented here. Despite the fact that SOD1G37R;TH mice had 80% of ventral root axons with diameters below this putative threshold of vulnerability, their life span was comparable to SOD1G37R mice having normal NF levels and axon calibers (Fig. 6). Moreover, the number and caliber of distribution of surviving axons at the end stage of disease were identical in SOD1G37R and SOD1G37R;TH mice (Fig. 3C). These results show that reduction of axonal caliber in TH mice did not alleviate degeneration of motor neurons at risk in disease caused by mutant SOD1. Other mechanisms could then account for the increased resistance of motor axons after axotomy in SOD1G93A mice (45). For instance, astrocytes and microglia activation in mice expressing mutant SOD1 (26–28, 46) may enhance levels of cytokines in the spinal cord. A recent study suggests a beneficial role for acute inflammation and gliosis in contused spinal cord and axotomized facial nucleus (47).
Our data suggest that a high NF content does not account for the selective vulnerability of motor neurons in disease induced by mutant SOD1. However, this is not to imply that NF proteins are not involved in ALS. The discovery of mutations in the NF-H gene in some ALS cases (21–23) and of a NF-L mutation in some cases of Charcot–Marie–Toot disease (48) provided compelling evidence for NF involvement in neurodegenerative disorders. Moreover, our recent studies with a new mouse model of motor neuron disease emphasized the importance of NF subunit stoichiometry and of interactions of NF proteins with peripherin, a type III intermediate filament (IF) protein found in motor neurons (49). We showed that the NF-M and NF-H proteins, even at low levels, can interact with peripherin to form noxious inclusion bodies similar to those found in human ALS (50, 51). In addition, the death of motor neurons in transgenic mice overexpressing peripherin was precipitated by NF-L deficiency, a phenomenon associated with human ALS (52). To our knowledge, coexpression of peripherin together with NF proteins in adult is a unique feature of motor neurons. This characteristic, which constitutes a risk for development of toxic IF inclusion bodies, may contribute in part to the selective vulnerability of motor neurons in ALS.
Acknowledgments
The technical help of P. Hince, G. Gagnon, M. Attiwell, and D. Houle is gratefully acknowledged. We thank Dr. Q. Zhu, Dr. C. Lampron, and S. Couillard-Després for helpful discussions and Drs. D. L. Price (Johns Hopkins, Baltimore, MD) and D. W. Cleveland (Ludwig Institute, La Jolla, CA) for the gift of the SOD1G37R mouse lines. This work was supported by the ALS Association of U.S.A., the ALS Society of Canada, and the Canadian Institutes for Health Research (CIHR). M.D.N. is a recipient of a MRC studentship and a K. M. Hunter/MRC doctoral scholarship. J.-P.J. holds a CIHR senior scholarship.
Abbreviations
- SOD1
Cu/Zn superoxide dismutase
- ALS
amyotrophic lateral sclerosis
- NF
neurofilament
- TH
triple NF heterozygous knockout
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cleveland D W. Neuron. 1999;24:515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Alexianu M E, Robbins E, Carswell S, Appel S H. J Neurosci Res. 1998;51:58–66. doi: 10.1002/(SICI)1097-4547(19980101)51:1<58::AID-JNR6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Alexianu M E, Ho B K, Mohamed A H, La Bella V, Smith R G, Appel S H. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 4.Ono S, Imai T, Munakata S, Takahashi K, Kanda F, Hashimoto K, Yamano T, Shimizu N, Nagao K, Yamauchi M. J Neurol Sci. 1998;160:140–147. doi: 10.1016/s0022-510x(98)00223-8. [DOI] [PubMed] [Google Scholar]
- 5.Williams T L, Day N C, Ince P G, Kamboj R K, Shaw P J. Ann Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]
- 6.Annesser J M, Borasio G D, Berthele A, Zieglgansberger W, Tolle T R. Neurobiol Dis. 1999;6:140–147. doi: 10.1006/nbdi.1999.0237. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Althaus J S, Ellerbrock B R, Becker D A, Gurney M E. Ann Neurol. 1998;44:763–770. doi: 10.1002/ana.410440510. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura Y, Dyck P J, Shimono M, Okazaki H, Takeishi J, Doi H. J Neuropathol Exp Neurol. 1981;40:667–675. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sobue G, Matsuoka Y, Mukai E, Takayanagi T, Sobue I. J Neurol Sci. 1981;50:413–421. doi: 10.1016/0022-510x(81)90153-2. [DOI] [PubMed] [Google Scholar]
- 10.Sobue G, Hashizume Y, Mitsuma T, Takahashi A. Neurology. 1987;37:529–532. doi: 10.1212/wnl.37.3.529. [DOI] [PubMed] [Google Scholar]
- 11.Julien J-P. Curr Opin Neurobiol. 1999;9:554–560. doi: 10.1016/S0959-4388(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee M K, Cleveland D W. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 13.Jacomy H, Zhu Q, Couillard-Després J-S, Beaulieu J-M, Julien J-P. J Neurochem. 1999;73:972–984. doi: 10.1046/j.1471-4159.1999.0730972.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee M K, Xu Z, Wong P C, Cleveland D W. J Cell Biol. 1993;122:1337–1350. doi: 10.1083/jcb.122.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasaki H, Itakura C, Mizutani M. Acta Neuropathol. 1991;82:427–434. doi: 10.1007/BF00293376. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Q, Couillard-Després S-D, Julien J-P. Exp Neurol. 1997;148:299–316. doi: 10.1006/exnr.1997.6654. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter S. Neurology. 1968;18:841–851. doi: 10.1212/wnl.18.9.841. [DOI] [PubMed] [Google Scholar]
- 18.Rouleau G A, Clarke A W, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien J-P, Figlewicz D A. Ann Neurol. 1996;39:128–131. doi: 10.1002/ana.410390119. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Cork L C, Griffin J W, Cleveland D W. Cell. 1993;73:23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 20.Côté F, Collard J F, Julien J P. Cell. 1993;73:35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 21.Figlewicz D A, Krizus A, Martinoli M G, Meininger V, Dib M, Rouleau G A, Julien J-P. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 22.Al-Chalabi A, Andersen P M, Nilsson P, Chioza B, Andersson J L, Russ C, Shaw C E, Powell J F, Leigh P N. Hum Mol Genet. 1999;8:157–164. doi: 10.1093/hmg/8.2.157. [DOI] [PubMed] [Google Scholar]
- 23.Tomkins J, Usher P, Slade J Y, Ince P G, Curtis A, Bushby K, Shaw P J. NeuroReport. 1998;9:3967–3970. doi: 10.1097/00001756-199812010-00036. [DOI] [PubMed] [Google Scholar]
- 24.Gurney M, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H-X. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 25.Tu P H, Raju P, Robinson K A, Gurney M E, Trojanowski J Q, Lee V M-Y. Proc Natl Acad Sci USA. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong P C, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 27.Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, Cleveland D W. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 28.Morrison B M, Gordon J W, Ripps M E, Morrison J H. J Comp Neurol. 1996;373:619–631. doi: 10.1002/(SICI)1096-9861(19960930)373:4<619::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Rheaume A G, Eliott J L, Hoffman E K, Kowall N W, Ferrante R J, Siwek D F, Wilcox H M, Flood D G, Beal M F, Brown R H, Jr, et al. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 30.Bruijn L I, Houseweart M K, Kato S, Anderson K L, Anderson S D, Ohama E, Reaume A G, Scott R W, Cleveland D W. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 31.Williamson T L, Bruijn L I, Zhu Q, Anderson K L, Anderson S D, Julien J-P, Cleveland D W. Proc Natl Acad Sci USA. 1998;95:9631–9636. doi: 10.1073/pnas.95.16.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Couillard-Després J-S, Zhu Q, Wong P C, Price D L, Cleveland D W, Julien J-P. Proc Natl Acad Sci USA. 1998;95:9626–9630. doi: 10.1073/pnas.95.16.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Q, Lindenbaum M, Levavasseur F, Jacomy H, Julien J P. J Cell Biol. 1998;143:183–193. doi: 10.1083/jcb.143.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julien J P, Tretjakoff I, Beaudet L, Peterson A C. Genes Dev. 1987;1:1085–1095. doi: 10.1101/gad.1.10.1085. [DOI] [PubMed] [Google Scholar]
- 35.Eyer J, Cleveland D W, Wong P C, Peterson A C. Nature (London) 1998;391:584–587. doi: 10.1038/35378. [DOI] [PubMed] [Google Scholar]
- 36.Meier J, Couillard-Després S, Jacomy H, Gravel C, Julien J-P. J Neuropathol Exp Neurol. 1999;58:1099–1110. [PubMed] [Google Scholar]
- 37.Crown J P, Sampson J B, Zhuang Y, Thompson J A, Beckman J S. J Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 38.Estevez A G, Crow J P, Sampson J B, Reiter C, Zhuang Y, Richardson G J, Tarpey M M, Barbeito L, Beckman J S. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 39.Elder G A, Friedrich V L, Jr, Margita A, Lazzarini R A. J Cell Biol. 1999;146:181–192. doi: 10.1083/jcb.146.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elder G A, Friedrich V L, Jr, Bosco P, Kang C, Gourov A, Tu P-H, Lee V M-Y, Lazzarini R A. J Cell Biol. 1998;143:727–739. doi: 10.1083/jcb.141.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elder G A, Friedrich V L, Jr, Kang C, Bosco P, Gourov A, Tu P-H, Zhang B, Lee V M-Y, Lazzarini R A. J Cell Biol. 1998;143:195–205. doi: 10.1083/jcb.143.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao M V, Houseweart M K, Williamson T L, Crawford T O, Folmer J, Cleveland D W. J Cell Biol. 1998;143:171–181. doi: 10.1083/jcb.143.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyer J, Peterson A C. Neuron. 1994;12:389–405. doi: 10.1016/0896-6273(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 44.Kong J, Tung V W-Y, Aghajanian J, Xu Z. J Cell Biol. 1998;140:1167–1176. doi: 10.1083/jcb.140.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong J, Xu Z. J Comp Neurol. 1999;412:373–380. doi: 10.1002/(sici)1096-9861(19990920)412:2<373::aid-cne13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 46.Almer G, Vukosavic S, Romero N, Przedborski S. J Neurochem. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- 47.Streit W J, Semple-Rowland S L, Hurley S D, Miller R C, Popovich P G, Stokes B T. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- 48.Mersinova I V, Perepelov A V, Polyakov A V, Sitnikov V F, Dadali E L, Oparin R B, Petrin A N, Evgrafov O V. Am J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulieu J-M, Nguyen M D, Julien J-P. J Cell Biol. 1999;147:531–544. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbo M, Hays A P. J Neuropathol Exp Neurol. 1992;51:531–537. doi: 10.1097/00005072-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Migheli A, Pezzulo T, Attanasio A, Schiffer D. Lab Invest. 1993;68:185–191. [PubMed] [Google Scholar]
- 52.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville M J, Percy M E. J Neuropathol Exp Neurol. 1994;53:221–230. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]