Abstract
We have investigated the mechanism underlying the modulation of the cardiac L-type Ca2+ current by protein kinase C (PKC). Using the patch-clamp technique, we found that PKC activation by 4-α-phorbol 12-myristate 13-acetate (PMA) or rac-1-oleyl-2-acetylglycerol (OAG) caused a substantial reduction in Ba2+ current through Cav1.2 channels composed of α11.2, β1b, and α2δ1 subunits expressed in tsA-201 cells. In contrast, Ba2+ current through a cloned brain isoform of the Cav1.2 channel (rbC-II) was unaffected by PKC activation. Two potential sites of PKC phosphorylation are present at positions 27 and 31 in the cardiac form of Cav1.2, but not in the brain form. Deletion of N-terminal residues 2–46 prevented PKC inhibition. Conversion of the threonines at positions 27 and 31 to alanine also abolished the PKC sensitivity of Cav1.2. Mutant Cav1.2 channels in which the threonines were converted singly to alanines were also insensitive to PKC modulation, suggesting that phosphorylation of both residues is required for PKC-dependent modulation. Consistent with this, mutating each of the threonines individually to aspartate in separate mutants restored the PKC sensitivity of Cav1.2, indicating that a change in net charge by phosphorylation of both sites is responsible for inhibition. Our results define the molecular basis for inhibition of cardiac Cav1.2 channels by the PKC pathway.
Calcium entering cardiac myocytes through voltage-gated L-type Ca2+ channels underlies the plateau of the action potential and initiates contraction (1, 2). L-type Ca2+ current is dynamically regulated by second messenger-activated protein kinases, phosphatases, and G proteins (1–3). Increased activity of L-type Ca2+ channels due to activation of β-adrenergic receptors and phosphorylation by cAMP-dependent protein kinase contributes to the increase in beating rate and contractile force upon activation of the sympathetic nervous system (1–3).
Molecular cloning studies have revealed that the Cav1.2 channels consisting of α11.2, β1 or β2, and α2δ subunits are responsible for L-type Ca2+ currents in cardiac myocytes (4–7). The α1 subunit forms the voltage-gated, Ca2+-selective pore and is the target for calcium antagonist drugs, whereas the β and α2δ subunits modulate channel function and increase functional expression (5–7). The α11.2 subunit contains approximately 2,100 amino acids organized in four homologous domains with six transmembrane segments and a membrane-reentrant pore loop in each (5–7). At each end of the protein are large intracellular N-terminal and C-terminal domains (5–7).
Regulation of L-type Ca2+ currents in cardiac and smooth muscle cells by receptor signaling pathways that activate protein kinase C (PKC) is well established. α-Adrenergic agonists (8), intracellular ATP (9), extracellular ATP (10), glucocorticoids (11), arginine-vasopressin (12), pituitary adenylate cyclase-activating polypeptide (13), and angiotensin II (14) all regulate L-type Ca2+ currents through the PKC pathway. In isolated cardiac myocytes, the effects of direct-acting PKC activators on the L-type Ca2+ current are varied and depend on the experimental preparation under study. The response to PKC activation can be a decrease (15–17) or a biphasic change (increase followed by a decrease) in Ca2+ current amplitude (18, 19).
Expression of a single combination of Ca2+ channel subunits in a heterologous cell type could in principle allow resolution of the distinct effects of PKC on Ca2+ channel activity. However, when expressed in Xenopus oocytes, Cav1.2 channels typically respond to PKC activation with an initial increase in current followed by a decrease (20–25). In contrast, we find that Cav1.2 channels expressed in the tsA-201 cell line of human embryonic kidney cells are inhibited by PKC, as observed in some studies of cardiac myocytes (15–17). Analysis of the molecular basis for this effect implicates phosphorylation of two threonine residues in the cardiac-specific N-terminal domain in inhibition of Cav1.2 channels by PKC.
Experimental Procedures
Construction of Mutants.
cDNAs encoding the wild-type α11.2, β1b, and α2δ subunits (5, 26–28) were subcloned into the pCDNA3.1 vector (Stratagene). The mutants were created by using overlap extension PCR mutagenesis with PFU Turbo (Stratagene). N-terminal PCR-generated mutagenic fragments of α11.2 were digested with HindIII and ClaI to enable a directional subcloning back into HindIII and ClaI predigested full-length α11.2 in pCDNA3.1. To delete amino acids 2 to 46, a mutagenic primer was designed that contains an internal HindIII restriction site and is composed of the 30 nucleotides flanking both sides of the nucleotides encoding amino acids 2 and 46 in α11.2. Mutations were confirmed by using automated cDNA sequencing (Applied Biosystems). DNA was prepared from transformed XL1-Blue competent Escherichia coli cells by using the Bio-Rad MaxiPrep Kit, following the manufacturer's recommended protocols.
Cell Culture.
tsA-201 cells, a subclone of the human embryonic kidney cell line HEK-293 that expresses the simian virus 40 T antigen (a gift of Robert Dubridge, Cell Genesis, Foster City, CA), were grown in DMEM/Ham's F-12 medium (Life Technologies, Grand Island, NY), supplemented with 10% (vol/vol) FBS (HyClone), and incubated at 37°C in 10% CO2.
Expression.
tsA-201 cells were grown to 90% confluence, suspended with trypsin/EDTA, and plated onto 35-mm culture dishes (Corning) at 40% confluence 24 h before transfection. Immediately before transfection, the medium was replaced with fresh DMEM/F-12 supplemented with serum and antibiotics, and the cells were transiently transfected with cDNAs encoding rabbit cardiac α11.2 (5) or rat brain α11.2 (26), β1b (27), and α2δ1 (28) subunits at a 1:1:1 molar ratio by using the calcium phosphate technique as described (29). In addition, cells were cotransfected at a 10-fold lower molar concentration with a CD-8 antigen (EBO-pCD-Leu2; American Type Culture Collection). The cells were incubated overnight at 37°C in 3% CO2. After 16 h, the medium was replaced with fresh DMEM/F-12, and the cells were allowed to recover for 9–12 h. After recovering from transfection the cells were suspended by using EDTA, plated in 35-mm dishes, and incubated at 37°C in 10% CO2 for 1–2 days before recordings.
Electrophysiology.
Immediately before recording, a 35-mm culture dish with transfected cells was stirred for 1 min with latex beads conjugated to an anti-CD8 antibody (Dynal, Oslo), which bound and decorated those cells that had been successfully transfected with the CD-8 receptor. The extracellular recording solution contained (in mM): 10 BaCl2, 140 Tris, 2 MgCl2, and 10 d-glucose titrated to pH 7.3 with MeSO4H. The intracellular solution contained (in mM): 130 N-methyl-d-glucamine, 60 Hepes, 5 MgATP, 1 MgCl2, and 10 EGTA titrated to pH 7.3 with MeSO4H. All experiments were performed at room temperature (20–23°C). Whole-cell patch clamp recordings were performed by using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) linked to a personal computer equipped with basic-fastlab acquisition software (Indec Systems, Capitola, CA). Patch pipettes were fabricated from VWR micropipettes and fire-polished. They had typical resistances of 2 MΩ in the solutions used in this study. After seal formation, the membrane beneath the pipette was ruptured and the pipette solution was allowed to dialyze into the cell for 1–2 min before recording. Currents were elicited by depolarization from a holding potential of −80 mV to various test potentials for 50–100 ms with an interpulse interval of 10 to 30 s. Voltage-dependent currents have been corrected for leak by using an on-line P/4 subtraction paradigm. Data were recorded at 10 kHz and filtered at 2 kHz before being stored directly on the computer hard drive. 4-α-Phorbol 12-myristate 13-acetate (PMA) and rac-1-oleyl-2-acetylglycerol (OAG) were prepared as 1 mM and 20 mM stock solutions, respectively, in DMSO and frozen in aliquots at −20°C. Immediately before use, a dilution of the required drug stock was prepared in extracellular recording solution such that the concentration of DMSO never exceeded 0.1%. At this concentration, there was no effect of DMSO on the Cav1.2 channel current. Data analysis was performed by using igor pro Version 3.12 (WaveMetrics, Lake Oswego, OR), and graphs were prepared by using origin Version 6.0 (Microcal, Northampton, MA). Error bars plotted represent the mean values ± standard error.
Results
Isoform-Specific Inhibition of Cardiac L-Type Ca2+ Channels by Activation of PKC.
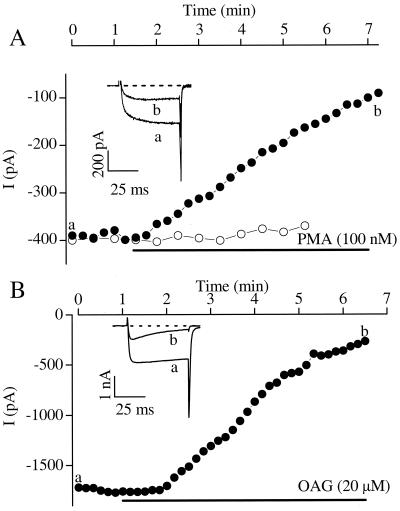
The L-type Ca2+ channel α11.2 subunit cloned from rabbit heart was expressed in tsA-201 cells together with the β1b and α2δ1 subunits. Ba2+ currents through the resulting L-type Ca2+ channels were decreased by activation of PKC with PMA (100 nM) (Fig. 1A). No transient increase in Ba2+ current was observed preceding the decrease. In contrast to these results with the cardiac Cav1.2 channel, an isoform of Cav1.2 cloned from rat brain (rbC-II; ref. 26) was unaffected by activation of PKC with PMA (Fig. 1A, open circles), as reported previously for expression of this isoform in Xenopus oocytes (30). These results suggest that the cardiac isoform of Cav1.2 is specifically sensitive to regulation by PKC.
Figure 1.
Inhibition of cardiac, but not brain, isoforms of Cav1.2 channels expressed in tsA-201 cells. Peak Ba2+ currents are plotted as a function of time of exposure to PKC activators for wild-type rabbit cardiac Cav1.2 (filled circles) or rat brain Cav1.2 (open circles). (A) 100 nM PMA. (B) 20 μM OAG. (Insets) Ba2+ currents through Cav1.2 channels were elicited by a depolarizing step from −80 mV to +10 mV before (a) and after (b) addition of PKC activator. Current traces (a, b) were taken from recordings at the time points indicated.
To test the specificity of the effects of PMA, we also activated PKC with OAG (20 μM). Activation with this diacylglycerol analog also inhibited Ba2+ current (Fig. 1B). The peak Ba2+ current was decreased without a shift in the voltage dependence of activation (Fig. 2 A and B). Peak Ba2+ current was reduced 61.5 ± 7.1% by PMA (n = 6) and 63.9 ± 8.7% by OAG (n = 5; Fig. 2C). The decrease in the Ba2+ current was not reversed by wash-out of the PKC activator during typical experiments of 8 to 15 min. The effects of PMA (100 nM) on the Cav1.2 channel current required PKC phosphorylation because the PKC inhibitor peptide PKC-I(19–36) blocked its effects (n = 2).
Figure 2.
Effects of PKC activators on peak Ba2+ currents and current–voltage relationships. (A) The peak current–voltage relationship plotted for a single cell studied as in Fig. 1, showing the control current (open circles) and the current in the presence of 100 nM PMA (filled squares). (B) Normalized current–voltage relationships for cells with or without exposure to 100 nM PMA for Ba2+ currents recorded as in Fig. 1. The current values were normalized for each cell to the peak value obtained from that cell's current–voltage relationship at ≈10 mV. Each point is the average of 3 to 6 experiments. (C) Mean changes in peak current amplitude in response to application of 20 μM OAG (−63.9 ± 8.7%; n = 5) or 100 nM PMA (−61.5 ± 7.1%; n = 6).
Effect of Mutations in the N-Terminal Domain on Regulation by PKC.
We focused initially on the proximal N-terminal domain as the site of
PKC regulation of the channel because the rat brain
Cav1.2 channel isoform rbC-II (26), which lacks a
segment within the first 46 amino acid residues in N-terminal domain of
the rabbit cardiac isoform (see sequences below), is insensitive to PKC
activators (30). Two potential PKC phosphorylation sites containing a
threonine followed at the +2 or +3 positions by one or more positively
charged residues are found in this segment (bold shaded residues in
sequences).
![]()
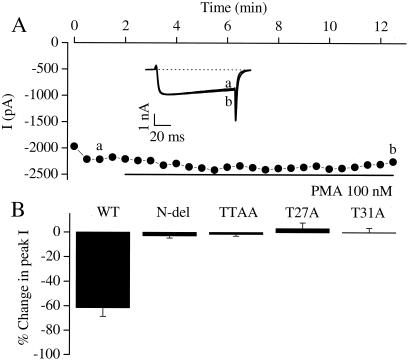
cDNA encoding a mutant Cav1.2 channel that lacked amino acid residues 2 to 46 in the N-terminal domain was constructed. This mutant formed functional Cav1.2 channels when expressed in tsA-201 cells, but truncation of the N terminus abolished the sensitivity to PMA (Fig. 3A). On average, the effect of PMA was reduced from a 61.5 ± 7.1% decrease in Ba2+ current to a 2.9 ± 1.8% decrease. This N-terminal segment contains two possible PKC phosphorylation sites, at threonine-27 and threonine-31. To determine whether these threonine residues are required for the PKC effects, a mutant (TTAA) was generated in which both threonines were mutated to alanine residues. The loss of the two threonines made the channel insensitive to PKC activators, suggesting that phosphorylation at these sites is required for inhibition of Cav1.2 channels by PKC (Fig. 3B).
Figure 3.
Effects of N-terminal mutations of threonine residues on PKC sensitivity of the Cav1.2 channel. (A) Peak Ba2+ currents recorded every 30 s. PMA was applied during the time indicated by the horizontal black bar. (Inset) Ba2+ currents elicited by a depolarizing step from −80 mV to +10 mV. Current traces such as those in the Inset were taken at time points a and b before and during exposure to PMA. (B) Mean changes in peak current amplitude in response to application of 100 nM PMA for wild-type Cav1.2 (WT) and several N-terminal mutants. The mean changes in current amplitude in response to PMA were as follows: Cav1.2 wild-type, −61.5 ± 7.1%, n = 6; N-terminal deletion [N-del (2–46)], −2.9 ± 1.8%, n = 3; TTAA, −1.5 ± 1.5%, n = 5; T27A, +3.2 ± 4.7%, n = 5; T31A, +0.2 ± 3.5%, n = 3.
Both Threonine-27 and Threonine-31 Are Required for PKC Regulation.
To examine the role of these two threonine residues separately, two further mutants were generated in which each of the threonines was mutated individually to alanine, and the resulting Cav1.2 channels were tested for sensitivity to PKC activators. Surprisingly, when either threonine-27 or threonine-31 was mutated to an alanine (T27A and T31A, respectively), the resulting single T → A mutant was insensitive to modulation by PKC activators (Fig. 3B). These data indicated that both threonines must be available for phosphorylation for PKC to inhibit the Cav1.2 channel.
Negative Charge at Positions 27 and 31 Supports Inhibition of Cav1.2.
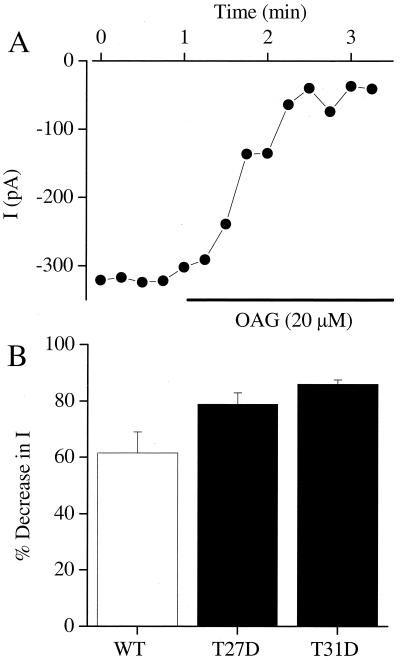
Phosphorylation of a neutral amino acid residue introduces negative charge at that site. We tested whether phosphorylation of either threonine could be mimicked by mutation to a negatively charged aspartate residue. When either threonine 27 or threonine 31 was converted to aspartate, the Ba2+ current remained sensitive to modulation by PKC, presumably by phosphorylation of the remaining site (Fig. 4). These data indicate that the net gain of negative charge at positions 27 and 31 within the N terminus region is a prerequisite for PKC-dependent modulation of the L-type Ca2+ channel. These results support a model in which phosphorylation of these two nearby threonine residues in the N-terminal domain of Cav1.2 inhibits its function.
Figure 4.
Effects of single aspartate substitutions in PKC sites on sensitivity of the Cav1.2 channel to PKC. (A) Peak Ba2+ currents for T31D elicited by a depolarizing step from −80 mV to +10 mV every 15 s. OAG (20 μM) was applied for the time indicated by the horizontal bar. (B) The average inhibition by OAG is shown for T27D (n = 4) and T31D (n = 5). For comparison, the effect of PMA on wild-type Cav1.2 (WT) is also plotted.
Discussion
PKC Activation Inhibits Cav1.2 Channels Expressed in tsA-201 Cells.
Modulation of L-type Ca2+ currents by PKC has been reported to result in a transient increase followed by a decrease, or a decrease in Ca2+ channel activity in isolated cardiac myocytes and smooth muscle cells (see Introduction). In this study, the response of the cardiac Cav1.2 channel to PKC activation in tsA-201 cells was consistently a progressive loss of Ba2+ current without any transient increase, consistent with results in some of the previous studies of cardiac myocytes (15–17). In our experiments, Ba2+ was used as the permeant ion, and intracellular Ca2+ transients were buffered by 10 mM EGTA. Because Ba2+ does not support PKC activity in purified enzyme preparations from rat brain (31, 32), the inhibitory effect of PKC activation on Cav1.2 channels is likely to be caused by activation of PKC by phorbol esters and OAG without an accompanying Ca2+ transient under our experimental conditions. Consistent with our results, allowing changes in the intracellular Ca2+ concentration by lowering the intracellular EGTA from 10 mM to 1 mM did not alter the response of the Ca2+ channel to PKC activators in cardiac myocytes (33).
The reasons for the different responses of Cav1.2 channels to PKC activation observed in different studies are unknown. One possible explanation is differential activation or membrane translocation of PKC isozymes in various cell preparations under the experimental conditions used in the different studies. PKC isozymes may phosphorylate different sites on the Cav1.2 channel, resulting in opposing functional effects. In adult rat cardiac myocytes, peptides corresponding to the C2 region of PKC abolished the PMA-induced decrease in Ca2+ current (17). Of the 11 cloned isoforms of PKC, only αI, βI, and βII have a C2 domain, so one of these isoforms is most likely responsible for the inhibitory effects of PKC on the Ca2+ current in this cell type. In the brain, species and age-related changes in PKC translocation have been suggested to arise as a result of differences in PKC anchoring (34). Thus, a combination of differential activation and translocation of PKC isozymes in different cell types and under different experimental conditions may lead to opposing effects on Cav1.2 channel activity. Isolation of the inhibitory effect of PKC under our experimental conditions in tsA-201 cells has allowed us to analyze the molecular basis for this form of regulation of Cav1.2 channel activity by PKC. Development of experimental conditions to isolate the activating effect of PKC will be necessary to analyze its molecular mechanism and to determine the relative importance of these opposing effects on channel activity in cardiac myocytes under different experimental conditions.
Two Amino Acid Residues Near the N Terminus Are Required for PKC Inhibition of Cardiac Cav1.2 Channels Expressed in tsA-201 Cells.
Our results identify two threonine residues in the cardiac L-type Cav1.2 channel that are necessary for inhibitory modulation by PKC. Both of these threonines are located in the N terminus of the Cav1.2 channel, at positions 27 and 31 in the cloned rabbit cardiac isoform. Both threonines must be available for phosphorylation, as mutation of either to an alanine rendered the channel insensitive to modulation by PKC. In addition, mutant channels in which either of these threonines was mutated singly to aspartate retained sensitivity to modulation by activators of PKC. Although we have not directly measured phosphorylation of these threonine residues, these results suggest that the local increase in negative charge in the N terminus of the channel after PKC phosphorylation is responsible for the inhibitory effect of PKC. If this region of the Cav1.2 channel is α-helical, these two threonine residues would be located close to each other in adjacent turns of the helix and would be presented together for phosphorylation and for interactions with other parts of the channel protein.
In contrast to our results, studies of a comparable cardiac Cav1.2 channel expressed in Xenopus oocytes showed biphasic regulation (24, 25). Mutants with a truncated N terminus lost sensitivity to regulation by PKC activators (25). However, a purified fusion protein containing residues 1–46 of Cav1.2, which contained the two threonines critical for the inhibitory effect of PKC on the Cav1.2 channel in our experiments in tsA-201 cells, was not phosphorylated in vitro. We assume that differences in the PKC isozymes in Xenopus oocytes and tsA-201 cells are responsible for these differences in regulatory effects.
Comparison of the Regulatory Effects of the N-Terminal and C-Terminal Domains of Cav1.2.
The C-terminal domain contains multiple sites of regulation of Cav1.2 channels. An EF-hand Ca2+-binding motif and a calmodulin-binding region are involved in Ca2+-dependent inactivation (35–38). A serine at position 1928 is an important site for phosphorylation by cAMP-dependent protein kinase (PKA) and consequent increase in Cav1.2 activity (39, 40). In vivo proteolytic processing truncates the C terminus and removes serine 1928 (39, 41, 42), and truncation of the C terminus increases Cav1.2 activity when expressed in Xenopus oocytes (43, 44). The function of the N terminus has been less well characterized. Similar to the C terminus, truncation greatly enhances the probability of channel opening in Xenopus oocytes and in HEK-293 cells (24, 45). Our results presented here show that phosphorylation of threonine residues in the N-terminal domain by PKC can inhibit channel activity. It is possible that the increase in Cav1.2 channel activity after truncation of the N-terminal domain reflects, in part, prevention of tonic inhibition by PKC phosphorylation. The N-terminal and C-terminal domains of Ca2+ channels are thought to interact in the folded structure of the protein. Therefore, these two structural components of the channel may form an extended regulatory domain that responds to changes in Ca2+, protein phosphorylation by both the PKA and PKC pathways, and regulated proteolysis. Integration of these different regulatory signals may provide sensitive modulation of channel activity in response to diverse patterns of cellular stimulation by hormones, neurotransmitters, and electrical activity.
Acknowledgments
This work was supported by National Institutes of Health Research Grant P01 HL44948 (to W.A.C.) and a postdoctoral fellowship from the Washington Affiliate of the American Heart Association (to D.M.).
Abbreviations
- PKC
protein kinase C
- PMA
4-α-phorbol 12-myristate 13-acetate
- OAG
rac-1-oleyl-2-acetylglycerol
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210384297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210384297
References
- 1.Reuter H. Nature (London) 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 2.McDonald T F, Pelzer S, Trautwein W, Pelzer D. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 3.Trautwein W, Hescheler J. Annu Rev Physiol. 1990;52:257–274. doi: 10.1146/annurev.ph.52.030190.001353. [DOI] [PubMed] [Google Scholar]
- 4.Ertel E A, Campbell K P, Harpold M M, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch T P, Tanabe T, Tsien R W, et al. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 5.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 6.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann F, Lacinova L, Klugbauer N. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 8.Woo S H, Lee C O. Pflügers Archiv. 1999;437:335–344. doi: 10.1007/s004240050787. [DOI] [PubMed] [Google Scholar]
- 9.McHugh D, Beech D J. J Physiol. 1997;500:311–317. doi: 10.1113/jphysiol.1997.sp022022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan Y W, Qi A-D. Can J Cardiol. 1997;13:1202–1211. [PubMed] [Google Scholar]
- 11.Kato H, Hayashi T, Koshino Y, Kutsumi Y, Nakai T, Miyabo S. Biochem Biophys Res Commun. 1992;188:934–941. doi: 10.1016/0006-291x(92)91145-g. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Hirano Y, Hiraoka M. Circ Res. 1995;76:592–599. doi: 10.1161/01.res.76.4.592. [DOI] [PubMed] [Google Scholar]
- 13.Chik C L, Li B, Ogiwara T, Ho A K, Karpinski E. FASEB J. 1996;10:1310–1317. doi: 10.1096/fasebj.10.11.8836045. [DOI] [PubMed] [Google Scholar]
- 14.Dosemeci A, Dhallon R S, Cohen N M, Lederer W J, Rogers T B. Circ Res. 1988;62:347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- 15.Satoh S. Gen Pharmacol. 1992;23:1097–1102. doi: 10.1016/0306-3623(92)90293-s. [DOI] [PubMed] [Google Scholar]
- 16.Satoh H. Jpn J Pharm. 1995;67:297–304. doi: 10.1254/jjp.67.297. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z-H, Johnson J A, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. Circ Res. 1997;80:720–729. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- 18.Lacerda A, Rampe D, Brown A M. Nature (London) 1988;335:249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- 19.Boixel C, Tessier S, Pansard Y, Lang-Lazdunski L, Mercadier J J, Hatem S N. Am J Physiol. 2000;278:H670–H676. doi: 10.1152/ajpheart.2000.278.2.H670. [DOI] [PubMed] [Google Scholar]
- 20.Singer-Lahat D, Gershon E, Lotan I, Hullin R, Biel B, Flockerzi V, Hofmann F, Dascal D. FEBS Lett. 1992;306:113–118. doi: 10.1016/0014-5793(92)80980-u. [DOI] [PubMed] [Google Scholar]
- 21.Bourinet E, Fournier P, Charnet P, Nargeot J. Pflügers Archiv. 1992;421:247–255. doi: 10.1007/BF00374834. [DOI] [PubMed] [Google Scholar]
- 22.Bouron A, Soldatov N M, Reuter H. FEBS Lett. 1995;377:159–162. doi: 10.1016/0014-5793(95)01327-x. [DOI] [PubMed] [Google Scholar]
- 23.Beech D J, Hering S, Kurka B, Sinnegger M J, Glossman H. XXXIII Int. Cong. Physiol. Sci. 1997. P002–P007. [Google Scholar]
- 24.Shistik E, Ivanina T, Blumenstein Y, Dascal N. J Biol Chem. 1998;273:17901–17909. doi: 10.1074/jbc.273.28.17901. [DOI] [PubMed] [Google Scholar]
- 25.Shistik E, Keren-Raifmann T, Idelson G H, Blumenstein Y, Dascal. N, Ivanina T. J Biol Chem. 1999;274:31145–31149. doi: 10.1074/jbc.274.44.31145. [DOI] [PubMed] [Google Scholar]
- 26.Snutch T P, Tomlinson W J, Leonard J P, Gilbert M M. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 27.Pragnell M, Sakamoto J, Jay S D, Campbell K P. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 28.Ellis S B, Williams M E, Ways N R, Brenner R, Sharp A H, Leung A T, Campbell K P, McKenna E, Koch W J, Hui A, et al. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 29.Margolskee R F, McHendry-Rinde B, Horn R. BioTechniques. 1994;15:906–911. [PubMed] [Google Scholar]
- 30.Stea A, Soong T W, Snutch T P. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. J Biol Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- 32.Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. J Biol Chem. 1979;254:3692–3695. [PubMed] [Google Scholar]
- 33.Tseng G-N, Boyden P A. Am J Physiol. 1991;261:H364–H379. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- 34.Pascale A, Govoni S, Battaini F. Mol Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602. [DOI] [PubMed] [Google Scholar]
- 35.Peterson B Z, Lee J S, Mulle J G, Wang Y, de Leon M, Yue D T. Biophys J. 2000;78:1906–1920. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson B Z, DeMaria C D, Adelman J P, Yue D T. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 37.Zuhlke R D, Pitt G S, Deisseroth K, Tsien R W, Reuter H. Nature (London) 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 38.Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L. Proc Natl Acad Sci U S A. 1999;96:2435–2438. doi: 10.1073/pnas.96.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jongh K S, Murphy B J, Colvin A A, Hell J W, Takahashi M, Catterall W A. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 40.Gao T, Yatani A, Dell'Acqua M L, Sako H, Green S A, Dascal N, Scott J D, Hosey M M. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 41.Hell J W, Westenbroek R E, Breeze L J, Wang K K W, Chavkin C, Catterall W A. Proc Natl Acad Sci USA. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerhardstein B L, Gao T, Bunemann M, Puri T S, Adair A, Ma H, Hosey M M. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 43.Wei X, Neely A, Lacerda A E, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. J Biol Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- 44.Klockner U, Mikala G, Varadi M, Schwartz A. J Biol Chem. 1995;270:17306–17310. doi: 10.1074/jbc.270.29.17306. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Neely A, Olcese R, Lang W, Stefani E, Stefani E, Birnbaumer L. Recept Channels. 1996;4:205–215. [PubMed] [Google Scholar]