Abstract
Melanocortin-4 receptor (Mc4r)-null mice exhibit late-onset obesity. To determine whether aberrant metabolism contributes to the obesity, food consumption by Mc4r-null mice was restricted to (pair-fed to) that consumed by wild-type (WT) mice. Pair-fed Mc4r-null females maintained body weights intermediate to that of WT and nonpair-fed Mc4r-null females, whereas pairfeeding normalized the body weights of Mc4r-null male mice. Fat pad and circulating leptin levels were elevated in both male and female pair-fed Mc4r-null mice compared with WT mice. Oxygen consumption of Mc4r-null mice with similar body weights as WT controls was reduced by 20%. Locomotor activity of young nonobese Mc4r-null males was significantly lower than that of WT males; however, locomotion of young nonobese females was normal. Core body temperature of Mc4r-null mice was normal, and they responded normally to cold exposure. Young nonobese Mc4r-null females were unable to induce uncoupling protein 1 (UCP1) in brown adipose tissue in response to peripheral leptin administration, whereas UCP1 mRNA was increased by 60% in the WT females. These results indicate that Mc4r deficiency enhances caloric efficiency, similar to that seen in the agouti obesity syndrome and in melanocortin-3 receptor-null mice.
Analyses of mutations at the agouti (A) locus along with pharmacological studies involving recombinant agouti protein have provided insights into the control of pigmentation and body weight. The agouti peptide is normally produced and secreted transiently in the skin, where it antagonizes the action of α-melanocyte stimulating hormone (α-MSH) acting on the melanocortin-1 receptor. This interaction results in deposition of bands of yellow pigment in otherwise black hairs, giving rise to the agouti coat color (1, 2). Several spontaneous chromosomal deletions that place the agouti gene under control of nearby ubiquitously expressed promoters have been identified in mice (3). Heterozygous mice with lethal yellow (Ay) and viable yellow (Avy) mutations have a syndrome known as the “yellow mouse syndrome,” characterized by maturity-onset obesity, increased linear growth, hyperleptinemia, hyperinsulinemia, noninsulin-dependent diabetes mellitus, and yellow fur (4). Homozygotes for these alleles die in utero because of a loss of essential genes (5).
Mice with multiple copies of the agouti gene expressed under the control of a skin-specific promoter manifested yellow fur but did not exhibit obesity-related phenotypes characteristic of the yellow mouse syndrome (6). Studies in vitro showed that agouti protein could also antagonize melanocortin-4 receptor (Mc4r) (7), which is expressed primarily in the nervous system with high levels in the hypothalamus (8, 9). Because the hypothalamus plays a critical role in the regulation of appetite and metabolic rate it was hypothesized that antagonism of Mc4r by agouti might be responsible for the obesity of Ay and Avy mice. Mice with targeted disruption of the Mc4r gene (Mc4r-null) reproduced the obesity phenotype of mice with the agouti syndrome, supporting this hypothesis (10).
Interruption of leptin or α-MSH signaling in the hypothalamus is thought to be the primary cause of the obesity exhibited by Ay (11) and Mc4r-null mice. Leptin is a hormone produced by adipose tissue that communicates the level of energy stores in the body to the brain. Animals that lack functional leptin, ob/ob mice, are morbidly obese and have a low metabolic rate. Leptin signals through the pro-opiomelanocortin (POMC)-containing neurons in the arcuate nucleus as well as other nuclei in the hypothalamus (12). POMC is the precursor of α-MSH, an agonist of Mc4r. These POMC-expressing neurons project to the lateral hypothalamus and paraventricular nucleus, where Mc4r is located. Activation of this leptin–melanocortin signaling pathway has been shown to affect food intake and metabolic rate. Adult Ay mice do not respond to s.c. leptin and require 100-fold more leptin to inhibit feeding (13). Likewise, adult Mc4r-null mice are resistant to the action of leptin (i.p. or centrally administered) with respect to food intake (14).
Both Mc4r-null (10) and Ay mice (4, 15–17) become hyperphagic; however, several experiments suggest that a metabolic component contributes to their obesity. Experiments in Avy mice (16) and melanocortin-3 receptor-null mice (18) indicate that these mutant strains of mice are more efficient at storing calories as adipose tissue than wild-type (WT) controls. Measurements of oxygen consumption indicate that obese Ay mice have a lower resting metabolic rate when tested between 2 and 12 mo of age (19), but not when tested at 6 wk of age. Finally, the metabolic rate of adult Mc4r-null, that were significantly heavier than control mice, was less than either lean controls or diet-induced obese controls when normalized to total body weight (20).
Signaling through the Mc4r activates the sympathetic nervous system (SNS) (21, 22), leading to increased release of norepinephrine, which activates a variety of metabolic processes, including thermogenesis in brown adipose tissue (BAT) by means of the induction of mitochondrial uncoupling protein 1 (UCP1). Induction of UCP1 generates heat by uncoupling oxidative phosphorylation in BAT, thereby helping rodents tolerate cold (23). UCP1 is also induced in BAT when rodents consume excess calories, in a process termed diet-induced thermogenesis (24). Diet-induced thermogenesis is also mediated by the SNS; however, its primary function is to expend excess calories as heat (24). Leptin can induce expression of UCP1 (25) in a manner that depends on activation of the SNS (26). One study indicated that the Mc4r is an important mediator of this response because central administration of leptin and a Mc4r antagonist completely inhibited the leptin-induced increase in UCP1 mRNA in BAT of lean rats (21). Another study found that administration of either leptin or a Mc4r agonist induced sympathetic nerve activity in BAT of lean rats. However, when leptin was administered with a Mc4r antagonist, sympathetic nerve activity in BAT was unchanged, suggesting that leptin can activate BAT independently of Mc4r (22).
If Mc4r is a critical mediator of metabolism and diet-induced thermogenesis, then Mc4r-null mice should be more metabolically efficient than control mice and should gain more weight (as adipose tissue) than control mice with the same food consumption (i.e., exhibit increased feed efficiency). We carried out long-term, pair-feeding experiments, which revealed that the Mc4r-null mice, like mice with the yellow mouse syndrome and melanocortin-3 receptor-null mice (18), show a predisposition to store calories as adipose tissue. To further characterize this metabolic defect, we tested oxygen consumption, locomotor activity, cold responsiveness, and the ability of leptin to induce UCP1 mRNA in Mc4r-null mice.
Materials and Methods
All animal procedures complied with National Institutes of Health guidelines and were approved by the University of Washington Animal Care Facility Committee.
Pair-Feeding Experiments.
F4 generation mice of mixed 129/Sv × C57BL/6J background heterozygous for the disrupted Mc4r allele were bred to generate Mc4r-null and WT littermate mice (10). Genotyping was performed as described (14). Mice were maintained on standard mouse chow (Harlin Teklad's standard laboratory mouse chow H8604) at 22°C on a 12-h light–dark cycle. Food intake of WT mice was measured daily starting at approximately 5 wk of age. Each day, group-housed male and female Mc4r-null mice were given the average amount of food consumed by their sex-matched WT littermates the previous day (27). Separate groups of group-housed male and female Mc4r-null mice were allowed ad libitum access to food. Body weights were measured weekly.
Fat Pad Measurements.
WT, pair-fed Mc4r-null and Mc4r-null mice with ad libitum access to food were killed at 38–46 wk of age. Inguinal, retroperitoneal, reproductive, scapular white adipose tissue, as well as scapular brown adipose tissue were immediately dissected and weighed.
Serum Measurements.
Trunk blood was collected from mice fed ad libitum in Microtainer Spin Tubes containing EDTA (Becton Dickinson) and immediately centrifuged at 2,000 rpm for 10 min at room temperature. Plasma was collected and frozen at −60°C. ELISA assays (Chrystal Chem, Chicago) were performed to measure leptin and insulin levels. Glucose levels were determined by using the YSI Select glucose analyzer (Yellow Springs Instruments).
Locomotor Activity.
Ambulatory activity of age-matched WT and Mc4r-null mice (4–8 wk of age) were measured in transparent Plexiglas cages (40 × 20 × 20-cm) surrounded by an aluminum frame equipped with infrared beams (San Diego Instruments). Mice were placed in the cages during the day, and beam breaks were measured for the first hour. Animals remained in the cages overnight with food and water available ad libitum, and light and dark cycle beam breaks were measured beginning on the second day. The number of consecutive beam breaks that occurred each hour during a 24-h period were measured and converted to meters traveled by using the distance between beams (8.8 cm) as a conversion factor.
Cold Tolerance.
Core body temperature of mice was measured at room temperature with a telethermometer (model 43TA, Yellow Springs Instruments), equipped with a rectal probe (model 402, Yellow Springs Instruments). The probe was inserted 5 cm into colon. Mice were then placed in a room maintained at 4°C, and body temperatures were measured similarly at 1, 4, 8, and 24 h after cold exposure.
Indirect O2 Consumption (VO2) Measurement.
WT and NPF Mc4r-null mice, 7.5–8.5 wk of age, were allowed to acclimatize to the experimental conditions by being placed in the chamber of an indirect open circuit calorimeter (Oxymax; Columbus Instruments) for 3 h on the day of assay. Measurements were carried out starting at approximately 12:00 h. The inlet fresh air flow-rate was 0.5 liter/min, the sample flow-rate was 0.5 liter/min, and the chamber was sampled for 60 s, with a resettling time of 60 s. Reported values are the means of 60 measurements over a 2-h period for each animal. There was no significant difference in oxygen consumption between females and males of each genotype; consequently, the results for these two groups were combined.
UCP1 mRNA Measurements.
Leptin (recombinant mouse; Calbiochem) injections were performed as described (25). Briefly, 5.5-wk-old WT and Mc4r-null female mice were injected once daily with 20 μg/g body weight recombinant mouse leptin or an equivalent volume of PBS vehicle, 3 h after the start of the light cycle for 3 days in a 22°C room. Three hours after the last injection, mice were killed and BAT was removed. Total nucleic acids were isolated from BAT, and relative levels of UCP 1 mRNA were quantified as described (28).
Statistical Analysis.
All values are reported as means ± SEM. Repeated measures ANOVA and Tukey's post hoc test were conducted for Fig. 1. ANOVA and Tukey's post hoc tests were conducted for Table 1 and Figs. 2 and 7. Student's t tests were conducted for Figs. 3–6.
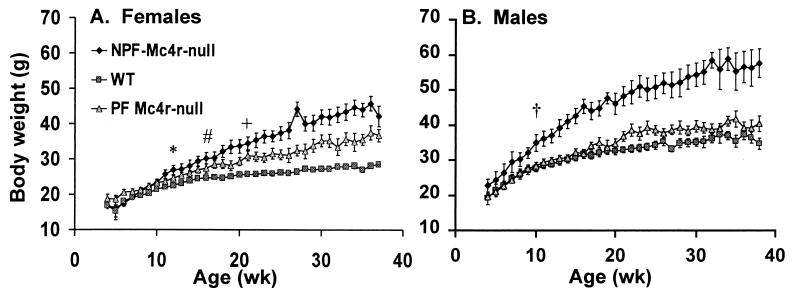
Figure 1.
Growth curves. (A) Weekly body weights of 12 WT, 14 PF Mc4r-null, and nine NPF Mc4r-null females. *, First week that body weight differed significantly between WT and NPF females (P < 0.05). #, First week that body weight differed significantly between WT and PF females (P < 0.05). +, First week that body weight differed significantly between PF and NPF females (P < 0.05). (B) Weekly body weights of 14 WT, nine PF Mc4r-null, and 12 NPF Mc4r-null males. †, First week that body weight of NPF Mc4r-null males differed significantly from WT or PF Mc4r-null males (P < 0.05).
Table 1.
Serum leptin, insulin, and glucose levels in WT, PF Mc4R-null, and NPF Mc4R-null mice
| Leptin (ng/ml) | Insulin (ng/ml) | Glucose (mg/dl) | |
|---|---|---|---|
| Female | |||
| WT (12) | 13.47 ± 2.15 | 1.29 ± 0.3 | 165.75 ± 7.0 |
| PF Mc4R-null (13) | 31.42 ± 5.69* | 2.08 ± 0.57 | 161.53 ± 4.79 |
| NPF Mc4R-null (10) | 87.16 ± 5.55*# | 16.34 ± 9.11*# | 294.4 ± 15.33*# |
| Male | |||
| WT (11) | 13.96 ± 2.87 | 4.16 ± 1.83 | 183.14 ± 9.07 |
| PF Mc4R-null (7) | 38.2 ± 4.90* | 12.11 ± 8.72 | 187.44 ± 14.11 |
| NPF Mc4R-null (10) | 75.67 ± 7.9*# | 75.42 ± 13.63* | 223.54 ± 22.1*# |
Trunk blood from WT, PF Mc4r-null, and NPF Mc4r-null mice was collected between 12:00 and 16:00 from animals with access to food ad libitum. Numbers in parentheses indicate the number of animals per group. Reported values are ± SEM. *, Significantly different from WT mice (P < 0.05).
, Significantly different from PF Mc4r-null mice (P < 0.05).
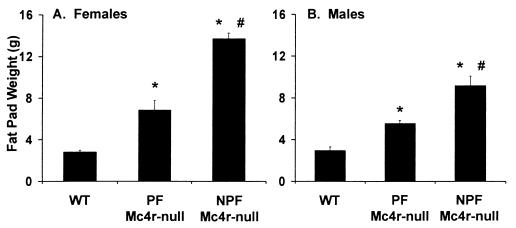
Figure 2.
Fat pad measurements. Adipose tissue from four fat depots (retroperitoneal, inguinal, reproductive, and scapular) were dissected and weighed. (A) Fat pad measurements from 12 WT, 14 PF Mc4r-null, and 10 NPF Mc4r-null female mice. (B) Fat pad measurements from14 WT, eight PF Mc4r-null, and 13 NPF Mc4r-null male mice. *, Significant difference from WT mice (P < 0.001). #, Significant difference between NPF Mc4r-null and PF mice (P < 0.001).
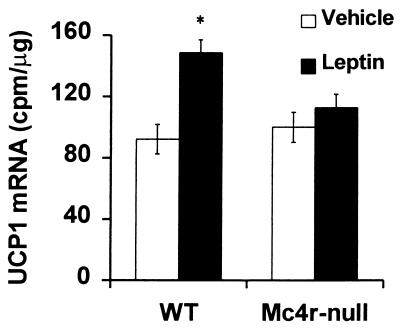
Figure 7.
Effect of leptin on UCP1 mRNA in BAT. Female WT (n = 6) and Mc4r-null (n = 6) mice were housed individually for 1 wk before treatment with leptin. The mice were injected daily with 20 μg/g body weight leptin or an equivalent volume of PBS vehicle for 3 days. Solution hybridization was used to measure UCP1 mRNA levels relative to total nucleic acid content. *, Significant increase relative to vehicle (P < 0.05).
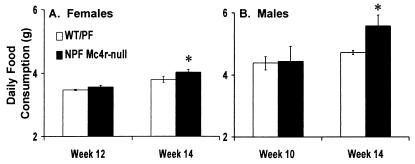
Figure 3.
Food consumption. Food consumption was measured for 5 days and reported as a daily average. Measurements were made at the first age when body weights were significantly different and at 14 wk. (A) Five WT and five NPF Mc4r-null females. (B) Five WT and five NPF Mc4r-null males. *, Significant difference from WT mice (P < 0.05).
Figure 6.
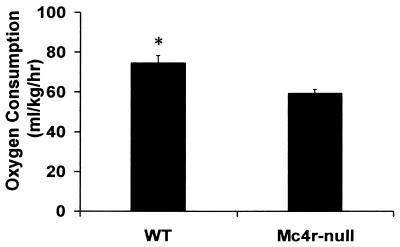
Oxygen consumption. Mice (7.5–8.5 wk old) were allowed to acclimate in the testing chamber for 2.5 h, then oxygen consumption was measured for 2 h at the same time each day (≈12:00–14:00 h). Total consumption was calculated for each mouse as indicated in Materials and Methods. The data represent the means from six trials with four WT mice and seven trials with four Mc4r-null mice. *, Significant difference between WT and NPF Mc4r-null mice (P < 0.003).
Results
Mc4r-null mice become obese and hyperphagic compared with WT mice; however, it is unclear whether disruption of melanocortin signaling primarily affects appetite, metabolism, or both. Therefore, to study the effects of appetite and metabolism on Mc4r-null mice, the daily food intake of young nonobese mice was restricted to that of WT mice, an experimental paradigm known as pair-feeding (25). Additionally, separate groups of male and female Mc4r-null littermates had ad libitum access to food. We have designated the animals pair-fed to the WT animals as PF Mc4r-null animals and those with access to food ad libitum as the nonpair-fed (NPF) Mc4r-null animals.
By 12 wk of age, the NPF Mc4r-null females had significantly greater average body weights (26.87 ± 1.35 g) than WT females (22.39 ± 0.38 g); their body weights continued to increase compared with WT females out to 37 wk, at which time they were 1.5-fold heavier (Fig. 1A). The PF Mc4r-null females had body weights intermediate between WT and NPF Mc4r-null females. They first attained greater average body weight than the WT female on week 17, and the significance of this difference was consistent after week 22. At 37 wk, PF Mc4r-null females had dissected body fat content that was intermediate between WT and NPF Mc4r-null females (Fig. 2A). The 3.5-g increase in fat pad mass observed in PF Mc4r-null females accounted for most of their body weight gain.
NPF Mc4r-null males also became 1.5-fold heavier than the WT males by 37 wk (Fig. 1B). In contrast to females, PF Mc4r-null males failed to gain weight relative to WT controls. Yet, like the PF Mc4r-null females, the PF Mc4r-null males had dissected body fat content that was significantly greater than WT males and intermediate between the WT and NPF Mc4r-null males (Fig. 2B). Reflective of their increased adiposity, both PF Mc4r-null females and males had greater serum leptin content than WT mice and lower serum leptin content than NPF Mc4r-null mice (Table 1). As previously reported, the insulin levels of the NPF Mc4r-null animals were significantly elevated over WT levels (10). Serum insulin levels in the PF Mc4r-null females and males were normalized, although there was a trend toward higher insulin levels in the PF Mc4r-null males. NPF Mc4r-null females were significantly hyperglycemic compared with controls (Table 1), in contrast to previous studies with Mc4r-null and Avy mice, in which only males were hyperglycemic (10, 29).
The increased body weight of the NPF Mc4r-null females at 12 wk of age, and of the NPF Mc4r-null males at 10 wk, occurred in the absence of any significant hyperphagia (Fig. 3). These results support a role for Mc4r in the regulation of metabolism.
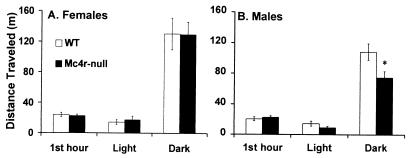
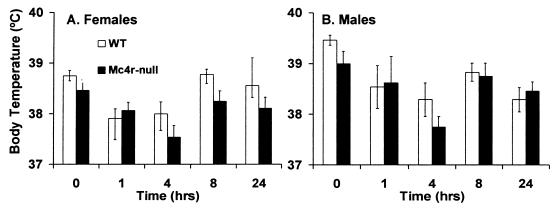
Mc4r could mediate a number of processes that would influence metabolism and cause Mc4r-null mice to have an increased propensity to store calories as adipose tissue. We investigated locomotor activity level, core body temperature (30), oxygen consumption (VO2), and the ability of leptin to regulate UCP1 expression (25). There was no difference in the distance traveled by lean Mc4r-null and WT females during any portion of the day or night (Fig. 4A). However, similar to findings by Chen et al. (20), Mc4r-null males traveled less than WT males during the dark cycle (Fig. 4B). The core body temperature in Mc4r-null mice was normal and they were able to thermoregulate at 4°C (Fig. 5), indicating that they could induce UCP1 expression when exposed to cold temperatures. At 7.5–8.5 wk of age, when NPF Mc4r-null animals and WT controls had similar body weights (average body weight WT = 19.48 ± 1.0 g; average body weight Mc4r-null = 21.06 ± 1.5 g), the NPF Mc4r-null animals consumed less oxygen (59.37 ± 1.99 ml/kg/h) than WT controls (79.55 ± 3.66 ml/kg/h). Total O2 consumption is shown in Fig. 6, but the same difference between genotypes was apparent when calculated as basal VO2 (data not shown). To determine whether leptin could induce UCP1 gene expression, leptin was administered (i.p.) for 3 days to young, nonobese Mc4r-null and WT females, and UCP1 mRNA was measured in BAT. Mc4r-null females were unable to induce UCP1 mRNA after leptin injection, whereas control animals exhibited a 60% increase in UCP1 mRNA in response to leptin treatment (Fig. 7).
Figure 4.
Ambulatory activity of Mc4r-null mice. Data are presented as meters traveled. The locomotion during the first hour represents activity in a novel environment; locomotion during 12-h light and dark cycles was recorded 24 h later. (A) Ambulatory activity of 4- to 8-wk-old WT (n = 10) and Mc4r-null (n = 11) females. (B) Ambulatory activity of 4- to 8-wk-old WT (n = 13) and Mc4r-null (n = 14) males. *, Significant difference between WT and Mc4r-null (P < 0.05).
Figure 5.
Core body temperature and cold responsiveness. Core body temperature of individually housed mice was measured before and at 1, 4, 8, and 24 h after placing mice at 4°C. (A) Eight WT and seven Mc4r-null female mice. (B) Six WT and six Mc4r-null male mice.
Discussion
The hypothalamus is thought to integrate many sensory and metabolic signals to regulate energy balance. Energy balance is maintained by controlling energy intake, as food, and energy expenditure, as physical activity and metabolism. Mc4r is an important component in this regulation as demonstrated by the phenotype of Mc4r-null mice and mice with the yellow mouse syndrome (4, 10). To investigate the contribution of metabolism, in the absence of hyperphagia, Mc4r-null mice were pair-fed to WT, sex-matched littermates. Our results indicate that Mc4r-signaling contributes to the regulation of metabolism. The PF Mc4r-null females became significantly heavier than their WT counterparts when given the same amount of food. Although the body weight of PF Mc4r-null males was not statistically different from WT males, there was a trend for the PF Mc4r-null males to be 2–3 g heavier than their WT counterparts. This body-weight difference was reflected in their fat pad weights, which were significantly elevated, mimicking the findings in Avy mice (16), and demonstrating a propensity to store calories as adipose tissue. Frigeri et al. (16) did not observe a sexually dimorphic effect in their study on efficiency of food utilization in Avy mice. The serum leptin levels of the PF Mc4r-null mice also reflected their increased adiposity. Pair-feeding normalized serum glucose and insulin levels, supporting reduced caloric intake as an effective treatment for type 2 diabetes mellitus.
The most compelling data suggesting that Mc4r functions in the regulation of metabolism is that the increased body weight of the NPF Mc4r-null females at 12 wk of age, and of the NPF Mc4r-null males at 10 wk, occurred in the absence of any significant hyperphagia (Fig. 3). It was not until 14 wk of age, when the NPF Mc4r-null females were 4.3 ± 1.4 g and males were 10.9 ± 1.8 g heavier than the WT controls, that they began to consume significantly more food. This result suggests that the metabolic deficit is the primary reason that Mc4r-null animals gain weight and that the hyperphagia occurs secondarily to support the animals increased body weight. This hypothesis is further supported by measurements of metabolic rate in young animals with similar body weights. We found that the Mc4r-null animals consumed 20% less oxygen than did the WT mice of the same weight (Fig. 6).
Mc4r-signaling could influence metabolism in a number of ways. Mc4r-null mice responded normally to cold temperatures; however, the young, lean Mc4r-null females did not induce UCP1 mRNA in response to leptin. Our results are consistent with those reported by Satoh et al. (21) who found that the induction of UCP1 by leptin (administered centrally) could be blocked by central administration of the Mc4r antagonist, SHU9119. Taken together, these results suggest that Mc4r is involved in mediating leptin activation of the SNS and subsequent diet-induced thermogenesis.
Leptin regulates both food intake and energy expenditure (31); however, it is unclear whether the effects of leptin on these processes occur by means of separate or common signaling pathways. Previously, we showed that, with respect to food intake, obese Mc4r-null mice were resistant to leptin, whereas young lean Mc4r-null mice were partially sensitive to leptin, suggesting that leptin resistance with respect to food intake in Mc4r-null mice is at least partially a consequence of the animals being obese (14). Here, we show that the inability of leptin to induce UCP1 mRNA in Mc4r-null females is evident when the mice are young and lean. Thus, the Mc4r-null mice are insensitive to leptin-mediated energy expenditure before they become completely resistant to leptin-induced inhibition of food intake. This result reinforces the idea that defective regulation of energy expenditure by Mc4r-null mice leads to obesity and that a compensatory increase in appetite occurs after the animals have increased body weights.
The data presented here indicate that Mc4r-null mice are more metabolically efficient than WT mice. A similar finding has recently been reported for melanocortin-3 receptor-null mice (18). Mc4r mutations in humans have been associated with a dominantly inherited form of obesity (32, 33). Many aspects of the regulation of energy homeostasis and the central melanocortin system appear to be similar in mouse and humans (reviewed in ref. 34). However it is unclear whether human beings with Mc4r mutations are more metabolically efficient. A recent report has indicated that Caucasians with heterozygous Mc4r mutations have a normal basal metabolic rate, whereas Indian/Pakistani subjects, with heterozygous or homozygous Mc4r mutations, show a trend toward negative deviations in basal metabolic rate (35).
Abbreviations
- A
agouti
- BAT
brown adipose tissue
- α-MSH
α-melanocyte stimulating hormone
- Mc4r
melanocortin-4 receptor
- NPF
nonpair-fed
- PF
pair-fed
- SNS
sympathetic nervous system
- UCP1
uncoupling protein 1
- WT
wild type
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220409497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220409497
References
- 1.Robbins L S, Nadeau J H, Johnson K R, Kelly M A, Roselli-Rehfuss L, Baack E, Mountjoy K G, Cone R D. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 2.Miltenberger R J, Mynatt R L, Wilkinson J E, Woychik R P. J Nutr. 1997;127:1902S–1907S. doi: 10.1093/jn/127.9.1902S. [DOI] [PubMed] [Google Scholar]
- 3.Bultman S J, Michaud E J, Woychik R P. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 4.Yen T T, Gill A M, Frigeri L G, Barsh G S, Wolff G L. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 5.Michaud E J, Bultman S J, Woychik R P. Genes Dev. 1993;7A:1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- 6.Kucera G T, Bortner D M, Rosenberg M P. Dev Biol. 1996;173:162–173. doi: 10.1006/dbio.1996.0014. [DOI] [PubMed] [Google Scholar]
- 7.Lu D, Willard D, Patel I R, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik R P, Wilkinson W O, Cone R D. Nature (London) 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 8.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson S J, DelValle J, Yamada T. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 9.Mountjoy K G, Mortrud M T, Low M J, Simerly R B, Cone R D. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 10.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R A, Boston B A, Cone R D, et al. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 11.Boston B A, Blaydon K M, Varnerin J, Cone R D. Science. 1997;278:1641–1644. doi: 10.1126/science.278.5343.1641. [DOI] [PubMed] [Google Scholar]
- 12.Cheung C C, Clifton D K, Steiner R A. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 13.Halaas J L, Boozer C, Blair-West J, Fidahusein N, Denton D A, Friedman J M. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh D J, Hollopeter G, Huszar D, Laufer R, Yagaloff K A, Fisher S L, Burn P, Palmiter R D. Nat Genet. 1999;21:122–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 15.Yen T T, McKee M M, Stamm M B. Int J Obes. 1984;8, Suppl. 1:65–78. [PubMed] [Google Scholar]
- 16.Frigeri L G, Wolff G L, Teguh C. Int J Obes. 1988;12:305–320. [PubMed] [Google Scholar]
- 17.Bray G A, Shimizu H, Retzios A D, Shargill N S, York D A. In: Obesity in Europe 88. Bjorntorp P, Rossner S, editors. London: John Libbey; 1988. pp. 259–270. [Google Scholar]
- 18.Chen A S, Marsh D J, Trumbauer M E, Frazier E G, Guan X, Yu H, Rosenblum C I, Vongs A, Yue F, Cao L, et al. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 19.Bartke A, Gorecki A. Am J Physiol. 1968;214:1250–1252. doi: 10.1152/ajplegacy.1968.214.6.1250. [DOI] [PubMed] [Google Scholar]
- 20.Chen A S, Metzger J M, Trumbauer M E, Guan X, Yu H, Frazier E G, Marsh D J, Forrest M J, Gopal-Truter S, Fisher J, et al. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 21.Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K. Neuroscience. 1998;249:107–110. doi: 10.1016/s0304-3940(98)00401-7. [DOI] [PubMed] [Google Scholar]
- 22.Haynes W G, Morgan D A, Walsh S A, Allyn M L, Sivitz W I. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsson A, Stadler U, Glotzer M A, Kozak L P. J Biol Chem. 1985;260:16250–16254. [PubMed] [Google Scholar]
- 24.Himms-Hagen J. Can J Biochem Cell Biol. 1983;62:610–617. doi: 10.1139/o84-081. [DOI] [PubMed] [Google Scholar]
- 25.Commins S P, Watson P M, Padgett M A, Dudley A, Argyropoulos G, Gettys T W. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 26.Commins S P, Marsh D J, Thomas S A, Watson P M, Padgett M A, Palmiter R D, Gettys T W. Endocrinology. 1999;140:4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 27.Levin N, Nelson C, Gurney A, Vandlen R, De Sauvage F. Proc Natl Acad Sci USA. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas S A, Palmiter R D. Nature (London) 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- 29.Frigeri L G, Wolff G L, Robel G. Endocrinology. 1983;113:2097–2105. doi: 10.1210/endo-113-6-2097. [DOI] [PubMed] [Google Scholar]
- 30.Kim J H, Mynatt R L, Moore J W, Woychik R P, Moustais N, Zemel M B. FASEB J. 1996;10:1646–1652. [PubMed] [Google Scholar]
- 31.Elmquist J K, Elias C F, Saper C B. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 32.Yeo G S H, Farooqi I S, Aminian S, Halsall D J, Stanhope R G, O'Rahilly S. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 33.Vaisse C, Clement K, Guy-Garand B, Philippe F. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 34.Cone R D. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 35.Farooqi I S, Yeo G S H, Keogh J M, Aminian S, Jebb S A, Butler G, Cheetham T, O'Rahilly S. J Clin Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]