Abstract
Plants require metals for essential functions ranging from respiration to photosynthesis. These metals also contribute to the nutritional value of plants for both humans and livestock. Additionally, plants have the ability to accumulate nonessential metals such as cadmium and lead, and this ability could be harnessed to remove pollutant metals from the environment. Designing a transporter that specifically accumulates certain cations while excluding others has exciting applications in all of these areas. The Arabidopsis root membrane protein IRT1 is likely to be responsible for uptake of iron from the soil. Like other Fe(II) transporters identified to date, IRT1 transports a variety of other cations, including the essential metals zinc and manganese as well as the toxic metal cadmium. By heterologous expression in yeast, we show here that the replacement of a glutamic acid residue at position 103 in wild-type IRT1 with alanine increases the substrate specificity of the transporter by selectively eliminating its ability to transport zinc. Two other mutations, replacing the aspartic acid residues at either positions 100 or 136 with alanine, also increase IRT1 metal selectivity by eliminating transport of both iron and manganese. A number of other conserved residues in or near transmembrane domains appear to be essential for all transport function. Therefore, this study identifies at least some of the residues important for substrate selection and transport in a protein belonging to the ZIP gene family, a large transporter family found in a wide variety of organisms.
The primary control point for metal ion homeostasis appears to be regulation of metal uptake across the plasma membrane. One important question that arises is whether there are separate transporters for each metal and, if not, which metals share the same transporter system. We have recently identified a group of metal transporters, the ZIP gene family, whose members have been implicated in the transport of a variety of metals (1). IRT1, the first ZIP gene to be identified, was discovered by functional complementation of the fet3 fet4 iron uptake mutant of Saccharomyces cerevisiae (2). IRT1 is also capable of complementing the metal uptake defects of the S. cerevisiae zrt1 zrt2 zinc uptake mutant and the S. cerevisiae smf1 manganese uptake mutant (3). This IRT1 complementation data, along with results from radioactive uptake assays (2, 3), demonstrate that IRT1 is capable of transporting Fe, Zn, and Mn; sensitivity and accumulation studies show that IRT1 can also transport cadmium (E. L. Connolly and M.L.G., unpublished data and this work). In this respect, IRT1 is similar to other iron transporters described to date (4, 5).
The broad cation range of IRT1 makes it an excellent model system for the study of residues involved metal recognition and transport. To determine the importance of individual amino acid side chains for metal transport, we substituted alanine for a number of amino acid residues that are highly conserved among ZIP family members and are predicted to be potential metal ligands. Here, we report on three of these mutations that confer altered metal specificity.
Methods
Yeast Strains and Growth Conditions.
Yeast strains were grown by using standard methods (6) except as follows: fet3 fet4 (7), zrt1 zrt2 (8) and smf1 (3) were grown as described (2, 9, 10). For cadmium sensitivity, wild-type S. cerevisiae (DY1457; MATα ade6 can1 his3 leu2 trp1 ura3) was grown on SD containing 0.2 μM CdSO4.
Point Mutation Construction and DNA Manipulations.
Point mutations were constructed by using either the transformer site-directed mutagenesis kit (CLONTECH) or by the method of Kunkel (6, 11). All other DNA manipulations were carried out by using standard techniques (6). Alanine substitutions were chosen as they yield a chemically consistent set of substitutions in which all side chain atoms beyond Cβ (and interactions made by these atoms) are eliminated. Alanine substitutions are also reported to have minimal adverse effects on protein folding (12). IRT1 was expressed from the phosphoglycerate kinase promoter in the yeast expression vector pFL61 (13).
Uptake Assays.
Iron uptake assays were performed with the indicated IRT1 allele in pFL61 in fet3 fet4 as described (2). 10 μM 55FeCl3 and 15 μM unlabeled FeCl3 were used for a final iron concentration of 25 μM, approximately four times the 6 ± 1 μM Km of IRT1 for iron (2). Uptake assays with E103A were also performed at iron concentrations ranging from 3 to 200 μM. Zinc uptake assays were performed with the indicated IRT1 allele in pFL61 in zrt1 zrt2 as described (14). A total of 10 μM 65ZnCl2 was used, which is approximately four times the 2.8 ± 0.6 μM Km of IRT1 for zinc (3).
Results and Discussion
Expression of IRT1 Variants in Yeast.
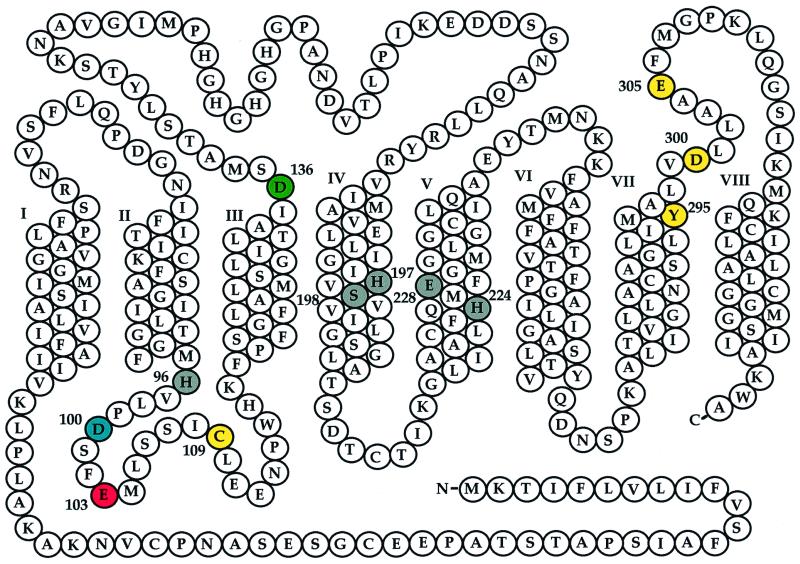
A number of potential metal ligands are highly conserved among ZIP family members (Fig. 1). These residues were individually replaced with alanine, and the resulting mutant alleles were expressed in different yeast strains to determine their ability to complement various yeast transport mutants or to confer cadmium sensitivity on wild-type yeast (Table 1 and Figs. 2 and 3). Glu-103 is predicted to lie in the extracellular loop between transmembrane domains II and III (Figs. 1 and 2). The substitution of an alanine residue for a glutamic acid residue at position 103 in IRT1 confers a dramatic phenotype, increasing the specificity of the transporter by eliminating its ability to transport zinc while not affecting its ability to transport iron, manganese, or cadmium. Asp-100 is predicted to be in the same extracellular loop as Glu-103, and its mutation also increases the specificity of the transporter, eliminating transport of iron and manganese but not of zinc and cadmium. It is intriguing that the ability to transport iron tracks with that of manganese but not with that of zinc. This observation suggests that iron and manganese transport may be mechanistically inseparable.
Figure 1.
Partial alignment of the amino acid sequence of selected ZIP family members. Functional transport data are available for the 11 ZIP family members shown here; to date, other members of this family have not been functionally characterized. Identical amino acids and conservative substitutions are shaded in gray; the residues targeted for mutation are shown in black. (A) Alignment of residues corresponding to IRT1 residues 77–149. (B) Alignment of residues corresponding to IRT1 residues 180–309. GenBank accession numbers are as follows: IRT1, U27590; LeIRT1, AF136579; LeIRT2, AF136580; RIT1, AF065444; ZIP1, AF033535; ZIP2, AF033536; ZIP3, ZF033537; ZRT1, P32804; ZRT2, Z73302; ZNT1, AF133267; hZIP2, AF186081.
Table 1.
Complementation and transport by IRT1 point mutants
| Substitution | Complements
defect in
|
|||
|---|---|---|---|---|
| Fe transport | Mn transport | Zn transport | Sensitive to Cd | |
| None (wild type) | + | + | + | + |
| H96A | − | − | − | − |
| D100A | − | − | + | + |
| E103A | + | + | − | + |
| D100A E103A | − | − | + | + |
| C109A | + | + | + | + |
| D136A | − | − | + | − |
| H197A | − | − | − | − |
| H197E | − | − | − | − |
| S198A | − | − | − | − |
| H224A | − | − | − | − |
| E228A | − | − | − | − |
| Y295A | + | +* | + | + |
| D300A | + | +* | + | + |
| E305A | + | +* | + | + |
All mutant IRT1 alleles were expressed in four different yeast strains (fet3 fet4, smf1, zrt1 zrt2, and wild type) to determine their ability to complement various yeast transport mutants and to confer cadmium sensitivity.
Colonies slightly smaller than wild type, indicating less manganese transport ability.
Figure 2.
Summary of point mutant phenotypes. A diagram of the IRT1 amino acid sequence is shown with predicted transmembrane domains in the center, numbered I–VIII, predicted cytoplasmic loops above, and predicted extracellular regions below. Residues shaded in pink (Glu-103) are those whose replacement with alanine leads to variants that transport Fe, Mn, and Cd but not Zn; those in blue (Asp-100) are residues whose replacement with alanine leads to variants that transport Zn and Cd but not Fe and Mn, and those in green (Asp-136) transport Zn and reduced levels of Cd. Residues whose replacement with alanine leads to variants with no transport activity are shown in gray (His-96, His-197, Ser-198, His-224, and Glu-228), and those with wild-type transport function are shown in gold (Cys-109, Tyr-295, Asp-300, and Glu-305).
Figure 3.
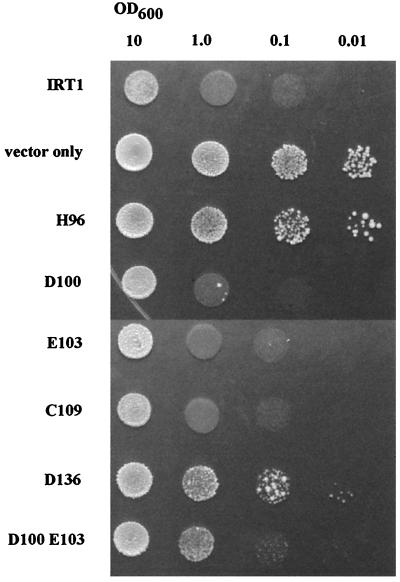
Growth of wild-type yeast cells expressing various IRT1 constructs on SD plates containing 0.2 μM CdSO4. Transformed strains were grown overnight in liquid SD medium. The cultures were adjusted to the OD levels indicated, and 20 μl was spotted onto plates. The plates were incubated at 30°C for 3 days before photography.
We reasoned that a variant carrying substitutions at both the Asp-100 and the Glu-103 positions might only transport cadmium, the one cation transported by both of the variants that carry the single mutations. Such a variant would be particularly useful in phytoremediation applications because, to date, no cadmium-specific transporters have been identified. There are several examples of cation transporters that transport cadmium as well as essential metal ions, as does IRT1. These include the wheat calcium transporter LCT1, the yeast low affinity iron transporter FET4, and lead- and zinc-effluxing transporters from Escherichia coli and Staphylococcus aureus (15–19). The strain carrying both the D100A and E103A mutations is less sensitive to cadmium than either single mutant (Fig. 3) and transports zinc but not iron or manganese. It is also interesting that Glu-103 is necessary for zinc transport in the context of the wild-type protein but not when combined with the D100A mutation.
Because Asp-100 and Glu-103 are both located in the extracellular loop between transmembrane domains II and III, we also substituted alanine for the cysteine at position 109; this cysteine is highly conserved among family members. This substitution had no apparent effect on transport. We did identify one other residue whose substitution resulted in a change in transporter specificity. Asp-136 is predicted to lie at the end of transmembrane domain III, close to the membrane on the cytoplasmic side (Figs. 1 and 2). The D136A mutation, like D100A, eliminates iron and manganese transport. However, unlike strains carrying the D100A allele, the strain carrying the D136A allele is no longer sensitive to 0.2 μM cadmium, indicating that the strain carrying this allele transports less cadmium than strains carrying either the wild-type IRT1 or the D100A allele (Fig. 3). Interestingly, the mutations that change the specificity of the IRT1 transporter are located in the first third of the protein, with two of the three mutations clustering in a single extracellular loop (Fig. 2). These results suggest that this loop is involved in binding the metal ion before transport.
His-96, His-197, His-224, and Glu-228 are all conserved, potential metal ligands predicted to lie in transmembrane domains. These residues are likely to be exposed on the surface of the pore or cavity formed by these domains and are good candidates for binding the substrate during transport. Perhaps not surprisingly, replacement of any of these residues with alanine eliminated all transporter function. Because transmembrane domain IV is predicted to be an amphipathic helix, we also replaced a highly conserved polar residue, a serine at position 198, with alanine. This residue is necessary for all transporter function and supports our previous prediction that His-197 and Ser-198 comprise part of a heavy metal binding site in the center of the membrane (20). Transmembrane domain VII is predicted to contain three highly conserved polar or charged residues: Tyr-295, Asp-300, and Glu-305. Replacement of any of these residues with alanine did not effect transporter function, suggesting these residues are not directly involved in passing substrates across the membrane.
Uptake Assays.
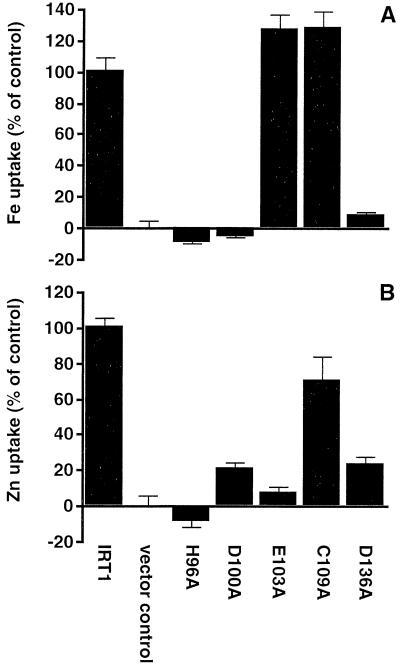
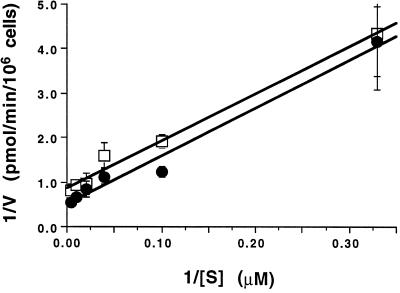
Complementation of yeast transport mutants is only a qualitative measure of transport ability. Therefore, uptake assays with radioactive iron or zinc were carried out with these mutants to quantify their transport activity. 55Fe uptake rates were examined in fet3 fet4 strains expressing various IRT1 alleles (Fig. 4A). Strains carrying the alleles that complement fet3 fet4 (E103A and C109A) show levels of uptake activity similar to strains carrying wild-type IRT1, whereas strains carrying the alleles that do not complement (H96A, D100A, and D136A) show no significant uptake activity over the level of the vector-only control strain. We also examined iron uptake over a range of concentrations for the strain carrying the E103A allele. These values were not significantly different from those of the strain carrying the wild-type allele, indicating that the Km and Vmax for these two transporters are also not significantly different (Fig. 5). Zinc transport activity of the same set of IRT1 mutant alleles was directly measured by examining 65Zn uptake rates in zrt1 zrt2 strains expressing those alleles. Fig. 4B shows the zinc uptake data; again, strains carrying alleles that do not complement zrt1 zrt2 (H96A and E103A) show uptake activity indistinguishable from the vector-only control strain. Strains carrying the alleles that do complement zrt1 zrt2 show varying levels of uptake activity. zrt1 zrt2 expressing C109A shows uptake activity similar to the strain expressing wild-type IRT1, whereas strains expressing D100A and D136A only show 20–25% of wild-type transport activity. Remarkably, this level of activity clearly is sufficient to complement the zrt1 zrt2 growth defect.
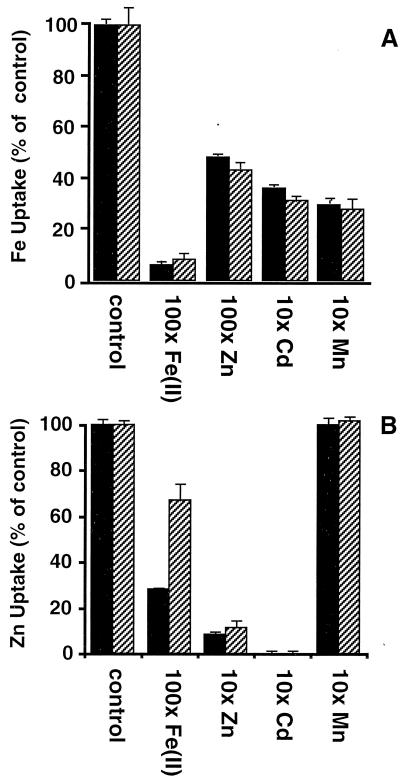
Figure 4.
(A) Iron uptake. Cell-associated counts were corrected for cell number, as measured by OD600, and normalized to control values. Therefore, small negative uptake numbers are possible for strains with no activity over the vector-only control. All assays were done in quadruplicate; the values and error bars are the mean and standard error, respectively. This experiment was performed four times; a representative data set is shown. (B) Zinc uptake. Values were determined as in A except that this experiment was performed three times; a representative data set is shown.
Figure 5.
Substrate dependence of iron uptake. Uptake assays were performed as described in Fig. 4A, varying the substrate concentration from 3 to 200 μM total Fe. Open squares represent IRT1 (y = 0.00086 + 0.011x; R2 = 0.99), and filled circles represent E103A (y = 0.00051 + 0.11x; R2 = 0.98). The data are shown as a double reciprocal Lineweaver–Burk plot. All assays were done in quadruplicate; the values and error bars are the mean and standard error, respectively. This experiment was performed twice; a representative data set is shown.
Competition Studies.
It has been previously shown that uptake of radiolabeled iron or zinc by IRT1 could be inhibited by the addition of excess iron, zinc, manganese, or cadmium (2, 3). To test if this inhibition was altered in the point mutants with altered specificities, iron uptake in the presence of excess iron, zinc, cadmium, or manganese was compared for the strain expressing the wild-type IRT1 protein and the strain expressing the E103A mutant protein that no longer transports zinc. In addition, zinc uptake activity in the presence of excess zinc, iron, cadmium, or manganese was also measured for the strain expressing wild-type IRT1 protein and for the strain expressing the D100A mutant protein, which no longer transports iron or manganese. As shown in Fig. 6B, the only significant difference between wild-type IRT1 protein and the mutants is the decreased competition of zinc uptake by the presence of excess iron in D100A. Zinc uptake in the strain carrying this allele was inhibited significantly less by the addition of 100× Fe(II) than was zinc uptake by the strain expressing the wild-type IRT1 allele. This parallels the transport phenotype and suggests that this residue is involved in the transport and/or binding of Fe(II) to IRT1. Iron transport by the strain expressing E103A and the strain expressing wild-type IRT1 are equally inhibited by zinc (Fig. 6A), indicating that either zinc inhibition is unrelated to cation binding for transport or that zinc can bind to the E103A mutant but not be transported.
Figure 6.
(A) Competition of iron uptake. The indicated amount of FeCl3, ZnSO4, CdSO4, or MnCl2 was added to the uptake assay as described in Fig. 4A. Solid bars represent IRT1, and striped bars represent E103A. This experiment was performed twice; a representative data set is shown. (B) Competition of zinc uptake. The indicated amount of FeCl3, ZnSO4, CdSO4, or MnCl2 was added to the uptake assay as described in Fig. 4B. Solid bars represent IRT1, and striped bars indicate D100A. This experiment was performed twice; a representative data set is shown.
Although there is one published report of changing the selectivity of the wheat high affinity K+-Na+ transporter with a single amino acid substitution (21), most examples of single amino acid substitutions affecting transporter activity either altered transporter activity with respect to one or more substrates or decreased transporter selectivity (22–25). The IRT1 examples discussed above demonstrate that we can alter the metal transport profile of a ZIP family member. One example of how such transporters could prove beneficial is in preventing iron-deficient plants from accumulating unwanted metals. It has been known for years that iron-deficient plants accumulate a variety of cations, including the toxic metal cadmium (26). We believe that such accumulation likely occurs via IRT1, which is turned on in roots in response to iron deficiency. The next step is to construct plants that no longer express a wild-type copy of IRT1 but rather express a version with an altered specificity and test such plants for metal accumulation. We also plan to use transporters with altered specificities in aid of phytoremediation (27, 28).
Acknowledgments
We thank Rob McClung and David Salt for their comments on the manuscript. This work was supported in part by a Department of Energy grant. E.E.R. is a Department of Energy, Energy Biosciences fellow of the Life Sciences Research Foundation.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.210214197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.210214197
References
- 1.Guerinot M L. Biochim Biophys Acta. 2000;1465:190–198. doi: 10.1016/s0005-2736(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 2.Eide D, Broderius M, Fett J, Guerinot M L. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korshunova Y, Eide D, Clark G, Guerinot M, Pakrasi H. Plant Mol Biol. 1999;40:37–44. doi: 10.1023/a:1026438615520. [DOI] [PubMed] [Google Scholar]
- 4.Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 5.Fleming M D, Trenor C C, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 2000. [Google Scholar]
- 7.Dix D R, Bridgham J T, Broderius M A, Byersdorfer C A, Eide D J. J Biol Chem. 1994;269:26092–26099. [PubMed] [Google Scholar]
- 8.Zhao H, Eide D. J Biol Chem. 1996;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- 9.Grotz N, Fox T, Connolly E L, Park W, Guerinot M L, Eide D. Proc Natl Acad Sci USA. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Supek F, Supekova L, Nelson H, Nelson N. Proc Natl Acad Sci USA. 1996;93:5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel T A. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett W F, Paoni N F, Keyt B A, Botstein D, Jones A J, Presta L, Wurm F M, Zoller M J. J Biol Chem. 1991;266:5191–5201. [PubMed] [Google Scholar]
- 13.Minet M, Dufour M E, Lacroute F. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Eide D. Proc Natl Acad Sci USA. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemens S, Antosiewicz D M, Ward J M, Schachtman D P, Schroeder J I. Proc Natl Acad Sci USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Kaplan J. J Biol Chem. 1998;273:22181–22187. doi: 10.1074/jbc.273.35.22181. [DOI] [PubMed] [Google Scholar]
- 17.Beard S J, Hashim R, Membrillo-Hernandez J, Hughes M N, Poole R K. Mol Microbiol. 1997;25:883–891. doi: 10.1111/j.1365-2958.1997.mmi518.x. [DOI] [PubMed] [Google Scholar]
- 18.Rensing C, Mitra B, Rosen B P. Proc Natl Acad Sci USA. 1997;94:14326–14331. doi: 10.1073/pnas.94.26.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rensing C, Sun Y, Mitra B, Rosen B P. J Biol Chem. 1998;273:32614–32617. doi: 10.1074/jbc.273.49.32614. [DOI] [PubMed] [Google Scholar]
- 20.Eng B H, Guerinot M L, Eide D, Saier M. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- 21.Rubio F, Gassmann W, Schroeder J I. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 22.Lu J M-Y, Bush D R. Proc Natl Acad Sci USA. 1998;95:9025–9030. doi: 10.1073/pnas.95.15.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morsomme P, de Kerchove d'Exaerde A, De Meester S, Thinés D, Goffeau A, Boutry M. EMBO J. 1996;15:5513–5526. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura R L, Anderson J A, Gaber R F. J Biol Chem. 1997;272:1011–1018. doi: 10.1074/jbc.272.2.1011. [DOI] [PubMed] [Google Scholar]
- 25.Will A, Caspari T, Tanner W. Proc Natl Acad Sci USA. 1994;91:10163–10167. doi: 10.1073/pnas.91.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen C, Fox T, Garvin D, Kochian L. Plant Physiol. 1998;116:1063–1072. doi: 10.1104/pp.116.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salt D E, Smith R D, Raskin I. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- 28.Meagher R. Curr Opin Plant Biol. 2000;3:153–162. doi: 10.1016/s1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]