Abstract
OBJECTIVES—Leber's
hereditary optic neuropathy (LHON) is a mitochondrial disease
leading to bilateral loss of central vision and severe optic nerve
atrophy. A subtype of LHON presents additional clinical and MRI aspects
indistinguishable from those of multiple sclerosis (MS) (LHON-MS). In
patients with LHON or LHON-MS, an assessment was made of
(a) the severity of optic nerve damage,
using MRI and magnetisation transfer imaging (MTI), and
(b) the presence and extent of macroscopic
and microscopic pathology in the brain and cervical cord, using MRI and
MT ratio (MTR) and mean diffusivity (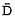 ) histogram analysis.
METHODS—Ten
patients with LHON, four with LHON-MS, and 20 age and sex matched
healthy controls were studied. For the optic nerve and the brain,
dual-echo turbo spin echo (TSE), T1 weighted spin echo, and MT images
were obtained. For the brain, fast fluid attenuated inversion recovery
(fast FLAIR) and diffusion weighted images were also obtained. For the
cervical cord, fast short tau inversion recovery (STIR) and MT images
were obtained. The volume and the average MTR value of both the optic
nerves were measured. MTR and
) histogram analysis.
METHODS—Ten
patients with LHON, four with LHON-MS, and 20 age and sex matched
healthy controls were studied. For the optic nerve and the brain,
dual-echo turbo spin echo (TSE), T1 weighted spin echo, and MT images
were obtained. For the brain, fast fluid attenuated inversion recovery
(fast FLAIR) and diffusion weighted images were also obtained. For the
cervical cord, fast short tau inversion recovery (STIR) and MT images
were obtained. The volume and the average MTR value of both the optic
nerves were measured. MTR and 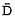 histograms of the normal appearing brain tissue (NABT) and MTR
histograms of the whole cervical cord tissue were created.
RESULTS—The mean
values of optic nerve volumes and MTR were significantly lower in
patients with LHON than in healthy controls. Mean NABT-MTR histogram
peak height was significantly lower in patients with LHON than in
controls, whereas no significant difference was found for any of the
cervical cord MTR histogram derived measures. Average
diffusivity
(
histograms of the normal appearing brain tissue (NABT) and MTR
histograms of the whole cervical cord tissue were created.
RESULTS—The mean
values of optic nerve volumes and MTR were significantly lower in
patients with LHON than in healthy controls. Mean NABT-MTR histogram
peak height was significantly lower in patients with LHON than in
controls, whereas no significant difference was found for any of the
cervical cord MTR histogram derived measures. Average
diffusivity
(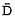 ) was higher in
patients with LHON than in controls. Optic nerve volume and MTR value
and mean NABT-MTR were lower in patients with LHON-MS than in those
with LHON.
CONCLUSIONS—The
severity of optic nerve pathology in LHON is measurable in vivo using
MRI and MTI. MTR and
) was higher in
patients with LHON than in controls. Optic nerve volume and MTR value
and mean NABT-MTR were lower in patients with LHON-MS than in those
with LHON.
CONCLUSIONS—The
severity of optic nerve pathology in LHON is measurable in vivo using
MRI and MTI. MTR and
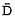 histogram
analysis suggests that microscopic brain damage occurs in LHON and that
it is more severe in the MS-like form of the disease.
histogram
analysis suggests that microscopic brain damage occurs in LHON and that
it is more severe in the MS-like form of the disease.
Full Text
The Full Text of this article is available as a PDF (179.3 KB).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. H., Blackwood W., Wilson J. Further clinical and pathological observations on Leber's optic atrophy. Brain. 1966 Mar;89(1):15–26. doi: 10.1093/brain/89.1.15. [DOI] [PubMed] [Google Scholar]
- Barbiroli B., Montagna P., Cortelli P., Iotti S., Lodi R., Barboni P., Monari L., Lugaresi E., Frassineti C., Zaniol P. Defective brain and muscle energy metabolism shown by in vivo 31P magnetic resonance spectroscopy in nonaffected carriers of 11778 mtDNA mutation. Neurology. 1995 Jul;45(7):1364–1369. doi: 10.1212/wnl.45.7.1364. [DOI] [PubMed] [Google Scholar]
- Boorstein J. M., Moonis G., Boorstein S. M., Patel Y. P., Culler A. S. Optic neuritis: imaging with magnetization transfer. AJR Am J Roentgenol. 1997 Dec;169(6):1709–1712. doi: 10.2214/ajr.169.6.9393194. [DOI] [PubMed] [Google Scholar]
- Campi A., Filippi M., Comi G., Martinelli V., Baratti C., Rovaris M., Scotti G. Acute transverse myelopathy: spinal and cranial MR study with clinical follow-up. AJNR Am J Neuroradiol. 1995 Jan;16(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- Cercignani M., Iannucci G., Rocca M. A., Comi G., Horsfield M. A., Filippi M. Pathologic damage in MS assessed by diffusion-weighted and magnetization transfer MRI. Neurology. 2000 Mar 14;54(5):1139–1144. doi: 10.1212/wnl.54.5.1139. [DOI] [PubMed] [Google Scholar]
- Cortelli P., Montagna P., Avoni P., Sangiorgi S., Bresolin N., Moggio M., Zaniol P., Mantovani V., Barboni P., Barbiroli B. Leber's hereditary optic neuropathy: genetic, biochemical, and phosphorus magnetic resonance spectroscopy study in an Italian family. Neurology. 1991 Aug;41(8):1211–1215. doi: 10.1212/wnl.41.8.1211. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L., Bozzali M., Cercignani M., Oldani A., Zucconi M., Filippi M. Magnetization transfer and diffusion-weighted imaging in nocturnal frontal lobe epilepsy. Neurology. 2000 Jun 27;54(12):2331–2333. doi: 10.1212/wnl.54.12.2331. [DOI] [PubMed] [Google Scholar]
- Filippi M., Bozzali M., Horsfield M. A., Rocca M. A., Sormani M. P., Iannucci G., Colombo B., Comi G. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology. 2000 Jan 11;54(1):207–213. doi: 10.1212/wnl.54.1.207. [DOI] [PubMed] [Google Scholar]
- Filippi M., Iannucci G., Tortorella C., Minicucci L., Horsfield M. A., Colombo B., Sormani M. P., Comi G. Comparison of MS clinical phenotypes using conventional and magnetization transfer MRI. Neurology. 1999 Feb;52(3):588–594. doi: 10.1212/wnl.52.3.588. [DOI] [PubMed] [Google Scholar]
- Filippi M., Yousry T., Baratti C., Horsfield M. A., Mammi S., Becker C., Voltz R., Spuler S., Campi A., Reiser M. F. Quantitative assessment of MRI lesion load in multiple sclerosis. A comparison of conventional spin-echo with fast fluid-attenuated inversion recovery. Brain. 1996 Aug;119(Pt 4):1349–1355. doi: 10.1093/brain/119.4.1349. [DOI] [PubMed] [Google Scholar]
- Flanigan K. M., Johns D. R. Association of the 11778 mitochondrial DNA mutation and demyelinating disease. Neurology. 1993 Dec;43(12):2720–2722. doi: 10.1212/wnl.43.12.2720. [DOI] [PubMed] [Google Scholar]
- Gawne-Cain M. L., O'Riordan J. I., Thompson A. J., Moseley I. F., Miller D. H. Multiple sclerosis lesion detection in the brain: a comparison of fast fluid-attenuated inversion recovery and conventional T2-weighted dual spin echo. Neurology. 1997 Aug;49(2):364–370. doi: 10.1212/wnl.49.2.364. [DOI] [PubMed] [Google Scholar]
- Harding A. E., Sweeney M. G., Miller D. H., Mumford C. J., Kellar-Wood H., Menard D., McDonald W. I., Compston D. A. Occurrence of a multiple sclerosis-like illness in women who have a Leber's hereditary optic neuropathy mitochondrial DNA mutation. Brain. 1992 Aug;115(Pt 4):979–989. doi: 10.1093/brain/115.4.979. [DOI] [PubMed] [Google Scholar]
- Horváth R., Abicht A., Shoubridge E. A., Karcagi V., Rózsa C., Komoly S., Lochmüller H. Leber's hereditary optic neuropathy presenting as multiple sclerosis-like disease of the CNS. J Neurol. 2000 Jan;247(1):65–67. doi: 10.1007/s004150050015. [DOI] [PubMed] [Google Scholar]
- Huoponen K., Vilkki J., Aula P., Nikoskelainen E. K., Savontaus M. L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991 Jun;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- Johns D. R., Smith K. H., Miller N. R. Leber's hereditary optic neuropathy. Clinical manifestations of the 3460 mutation. Arch Ophthalmol. 1992 Nov;110(11):1577–1581. doi: 10.1001/archopht.1992.01080230077025. [DOI] [PubMed] [Google Scholar]
- Kellar-Wood H., Robertson N., Govan G. G., Compston D. A., Harding A. E. Leber's hereditary optic neuropathy mitochondrial DNA mutations in multiple sclerosis. Ann Neurol. 1994 Jul;36(1):109–112. doi: 10.1002/ana.410360121. [DOI] [PubMed] [Google Scholar]
- Kermode A. G., Moseley I. F., Kendall B. E., Miller D. H., MacManus D. G., McDonald W. I. Magnetic resonance imaging in Leber's optic neuropathy. J Neurol Neurosurg Psychiatry. 1989 May;52(5):671–674. doi: 10.1136/jnnp.52.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Le Bihan D., Breton E., Lallemand D., Aubin M. L., Vignaud J., Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988 Aug;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- McGowan J. C., Filippi M., Campi A., Grossman R. I. Magnetisation transfer imaging: theory and application to multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998 May;64 (Suppl 1):S66–S69. [PubMed] [Google Scholar]
- Morrissey S. P., Borruat F. X., Miller D. H., Moseley I. F., Sweeney M. G., Govan G. G., Kelly M. A., Francis D. A., Harding A. E., McDonald W. I. Bilateral simultaneous optic neuropathy in adults: clinical, imaging, serological, and genetic studies. J Neurol Neurosurg Psychiatry. 1995 Jan;58(1):70–74. doi: 10.1136/jnnp.58.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman N. J., Lott M. T., Wallace D. C. The clinical characteristics of pedigrees of Leber's hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol. 1991 Jun 15;111(6):750–762. doi: 10.1016/s0002-9394(14)76784-4. [DOI] [PubMed] [Google Scholar]
- Nikoskelainen E. K., Marttila R. J., Huoponen K., Juvonen V., Lamminen T., Sonninen P., Savontaus M. L. Leber's "plus": neurological abnormalities in patients with Leber's hereditary optic neuropathy. J Neurol Neurosurg Psychiatry. 1995 Aug;59(2):160–164. doi: 10.1136/jnnp.59.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen N. K., Hansen A. W., Nørby S., Edal A. L., Jørgensen J. R., Rosenberg T. Leber's hereditary optic neuropathy associated with a disorder indistinguishable from multiple sclerosis in a male harbouring the mitochondrial DNA 11778 mutation. Acta Neurol Scand. 1995 May;91(5):326–329. doi: 10.1111/j.1600-0404.1995.tb07016.x. [DOI] [PubMed] [Google Scholar]
- Riordan-Eva P., Sanders M. D., Govan G. G., Sweeney M. G., Da Costa J., Harding A. E. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995 Apr;118(Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- Rocca M. A., Mastronardo G., Horsfield M. A., Pereira C., Iannucci G., Colombo B., Moiola L., Comi G., Filippi M. Comparison of three MR sequences for the detection of cervical cord lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol. 1999 Oct;20(9):1710–1716. [PMC free article] [PubMed] [Google Scholar]
- Rovaris M., Filippi M., Calori G., Rodegher M., Campi A., Colombo B., Comi G. Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol. 1997 Apr;244(4):266–270. doi: 10.1007/s004150050083. [DOI] [PubMed] [Google Scholar]
- Rovaris M., Filippi M. Magnetic resonance techniques to monitor disease evolution and treatment trial outcomes in multiple sclerosis. Curr Opin Neurol. 1999 Jun;12(3):337–344. doi: 10.1097/00019052-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Rovaris M., Viti B., Ciboddo G., Gerevini S., Capra R., Iannucci G., Comi G., Filippi M. Brain involvement in systemic immune mediated diseases: magnetic resonance and magnetisation transfer imaging study. J Neurol Neurosurg Psychiatry. 2000 Feb;68(2):170–177. doi: 10.1136/jnnp.68.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C., Hill D. L., Hawkes D. J. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997 Jan;24(1):25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Tartaglino L. M., Friedman D. P., Flanders A. E., Lublin F. D., Knobler R. L., Liem M. Multiple sclerosis in the spinal cord: MR appearance and correlation with clinical parameters. Radiology. 1995 Jun;195(3):725–732. doi: 10.1148/radiology.195.3.7754002. [DOI] [PubMed] [Google Scholar]
- Thorpe J. W., Barker G. J., Jones S. J., Moseley I., Losseff N., MacManus D. G., Webb S., Mortimer C., Plummer D. L., Tofts P. S. Magnetisation transfer ratios and transverse magnetisation decay curves in optic neuritis: correlation with clinical findings and electrophysiology. J Neurol Neurosurg Psychiatry. 1995 Nov;59(5):487–492. doi: 10.1136/jnnp.59.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C., Viti B., Bozzali M., Sormani M. P., Rizzo G., Gilardi M. F., Comi G., Filippi M. A magnetization transfer histogram study of normal-appearing brain tissue in MS. Neurology. 2000 Jan 11;54(1):186–193. doi: 10.1212/wnl.54.1.186. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- van Buchem M. A., McGowan J. C., Kolson D. L., Polansky M., Grossman R. I. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med. 1996 Oct;36(4):632–636. doi: 10.1002/mrm.1910360420. [DOI] [PubMed] [Google Scholar]
- van Waesberghe J. H., Kamphorst W., De Groot C. J., van Walderveen M. A., Castelijns J. A., Ravid R., Lycklama à Nijeholt G. J., van der Valk P., Polman C. H., Thompson A. J. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999 Nov;46(5):747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]


