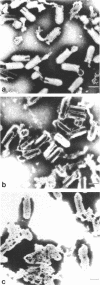
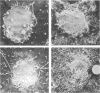
Abstract
Lipids in fresh human milk do not inactivate viruses but become antiviral after storage of the milk for a few days at 4 or 23 degrees C. The appearance of antiviral activity depends on active milk lipases and correlates with the release of free fatty acids in the milk. A number of fatty acids which are normal components of milk lipids were tested against enveloped viruses, i.e., vesicular stomatitis virus, herpes simplex virus, and visna virus, and against a nonenveloped virus, poliovirus. Short-chain and long-chain saturated fatty acids had no or a very small antiviral effect at the highest concentrations tested. Medium-chain saturated and long-chain unsaturated fatty acids, on the other hand, were all highly active against the enveloped viruses, although the fatty acid concentration required for maximum viral inactivation varied by as much as 20-fold. Monoglycerides of these fatty acids were also highly antiviral, in some instances at a concentration 10 times lower than that of the free fatty acids. None of the fatty acids inactivated poliovirus. Antiviral fatty acids were found to affect the viral envelope, causing leakage and at higher concentrations, a complete disintegration of the envelope and the viral particles. They also caused disintegration of the plasma membranes of tissue culture cells resulting in cell lysis and death. The same phenomenon occurred in cell cultures incubated with stored antiviral human milk. The antimicrobial effect of human milk lipids in vitro is therefore most likely caused by disintegration of cellular and viral membranes by fatty acids. Studies are needed to establish whether human milk lipids have an antimicrobial effect in the stomach and intestines of infants and to determine what role, if any, they play in protecting infants against gastrointestinal infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. The fusion of erythrocytes by fatty acids, esters, retinol and alpha-tocopherol. Biochem J. 1973 Sep;136(1):147–155. doi: 10.1042/bj1360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas-Rodriguez A., Smith H. W. The identification of the antimicrobial factors of the stomach contents of sucking rabbits. Biochem J. 1966 Jul;100(1):79–82. doi: 10.1042/bj1000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Cunningham A. S. Morbidity in breast-fed and artificially fed infants. II. J Pediatr. 1979 Nov;95(5 Pt 1):685–689. doi: 10.1016/s0022-3476(79)80711-8. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Diwan A. R., Halstead S. B. A lipid inhibitor of dengue virus in human colostrum and milk; with a note on the absence of anti-dengue secretory antibody. Arch Virol. 1975;47(1):3–10. doi: 10.1007/BF01315587. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H. Nonspecific antiviral substances in human milk active against arbovirus and murine leukemia virus. Cancer Res. 1974 Apr;34(4):712–715. [PubMed] [Google Scholar]
- Fredrikzon B., Hernell O., Bläckberg L., Olivecrona T. Bile salt-stimulated lipase in human milk: evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr Res. 1978 Nov;12(11):1048–1052. doi: 10.1203/00006450-197811000-00004. [DOI] [PubMed] [Google Scholar]
- Hernell O., Olivecrona T. Human milk lipases. I. Serum-stimulated lipase. J Lipid Res. 1974 Jul;15(4):367–374. [PubMed] [Google Scholar]
- Hernell O., Ward H., Bläckberg L., Pereira M. E. Killing of Giardia lamblia by human milk lipases: an effect mediated by lipolysis of milk lipids. J Infect Dis. 1986 Apr;153(4):715–720. doi: 10.1093/infdis/153.4.715. [DOI] [PubMed] [Google Scholar]
- Isaacs C. E., Thormar H., Pessolano T. Membrane-disruptive effect of human milk: inactivation of enveloped viruses. J Infect Dis. 1986 Dec;154(6):966–971. doi: 10.1093/infdis/154.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabara J. J. Lipids as host-resistance factors of human milk. Nutr Rev. 1980 Feb;38(2):65–73. doi: 10.1111/j.1753-4887.1980.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Lammi-Keefe C. J., Jensen R. G. Lipids in human milk: a review. 2: Composition and fat-soluble vitamins. J Pediatr Gastroenterol Nutr. 1984 Mar;3(2):172–198. doi: 10.1097/00005176-198403000-00004. [DOI] [PubMed] [Google Scholar]
- Larsen S. A., Jr, Homer D. R. Relation of breast versus bottle feeding to hospitalization for gastroenteritis in a middle-class U.S. population. J Pediatr. 1978 Mar;92(3):417–418. doi: 10.1016/s0022-3476(78)80430-2. [DOI] [PubMed] [Google Scholar]
- Matthews T. H., Nair C. D., Lawrence M. K., Tyrrell D. A. Antiviral activity in milk of possible clinical importance. Lancet. 1976 Dec 25;2(8000):1387–1389. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- Resta S., Luby J. P., Rosenfeld C. R., Siegel J. D. Isolation and propagation of a human enteric coronavirus. Science. 1985 Sep 6;229(4717):978–981. doi: 10.1126/science.2992091. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Charney J., Dion A. S., Moore D. H. Effect of human milk on the mouse mammary tumor virus. Cancer Res. 1973 Mar;33(3):626–629. [PubMed] [Google Scholar]
- Thormar H., Barshatzky M. R., Arnesen K., Kozlowski P. B. The emergence of antigenic variants is a rare event in long-term visna virus infection in vivo. J Gen Virol. 1983 Jul;64(Pt 7):1427–1432. doi: 10.1099/0022-1317-64-7-1427. [DOI] [PubMed] [Google Scholar]
- Welsh J. K., Arsenakis M., Coelen R. J., May J. T. Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis. 1979 Sep;140(3):322–328. doi: 10.1093/infdis/140.3.322. [DOI] [PubMed] [Google Scholar]
- Welsh J. K., Skurrie I. J., May J. T. Use of Semliki forest virus to identify lipid-mediated antiviral activity and anti-alphavirus immunoglobulin A in human milk. Infect Immun. 1978 Feb;19(2):395–401. doi: 10.1128/iai.19.2.395-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]