Abstract
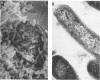
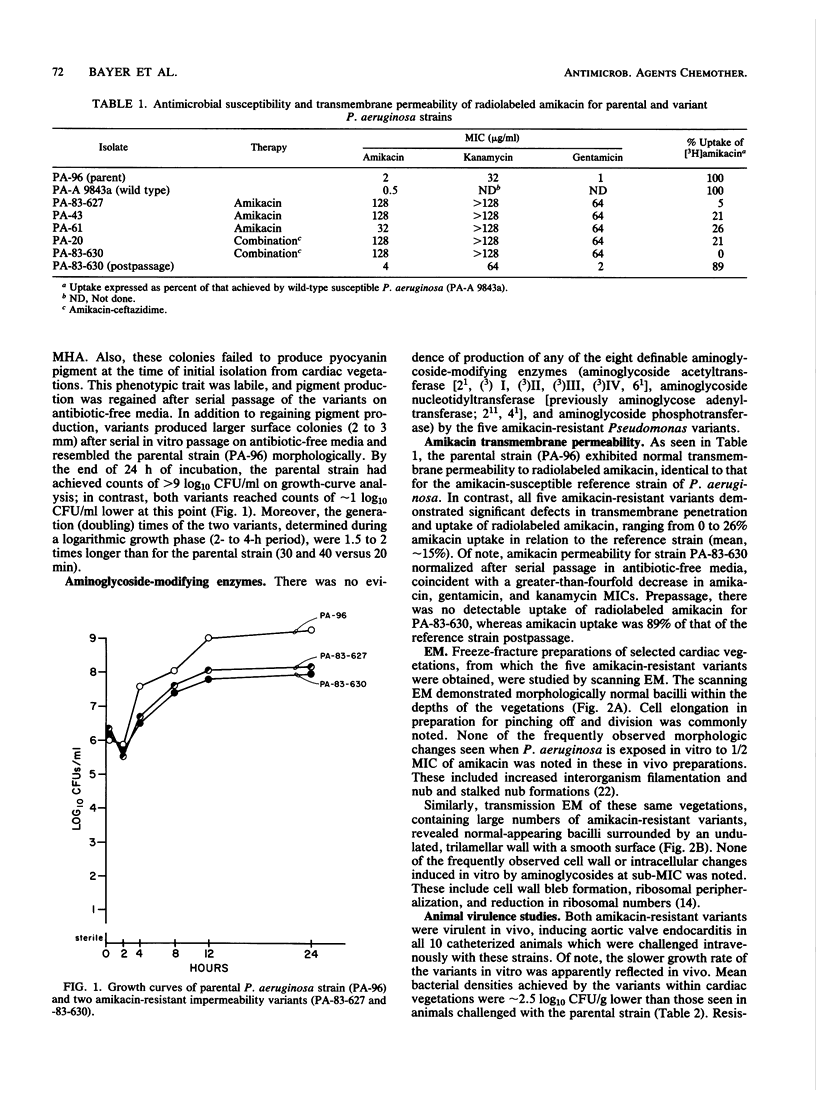
We characterized five amikacin-resistant variants of Pseudomonas aeruginosa isolated from aortic valve vegetations during unsuccessful therapy of experimental endocarditis. These organisms were cross resistant to other aminoglycosides. No aminoglycoside-modifying enzymes were produced by these strains. However, all five variants demonstrated significant defects in permeability and intracellular uptake of [3H]amikacin when compared with the amikacin-susceptible parental strain (0 to 26% of that of the parental strain; mean, approximately 15%). The permeability defects were unstable in vitro, with normalization after serial passage in antibiotic-free media. The variants grew as nonpigmented, small-colony types, with in vitro generation times approximately 1.5 to 2 times longer than that of the parental strain (30 to 40 versus 20 min, respectively). Two impermeability variants were compared with the parental strain for ability to induce experimental endocarditis in rabbits with aortic catheters. Both variants were virulent in vivo; however, mean bacterial densities in vegetations were approximately 2.5 log10 CFU/g lower in animals challenged with the variants than in animals challenged with the parental strain, probably reflecting a slower in vivo growth rate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G., Fekety F. R. Experimental endocarditis due to Pseudomonas aeruginosa. I. Description of a model. J Infect Dis. 1976 Jul;134(1):1–7. doi: 10.1093/infdis/134.1.1. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lam K., Norman D., Kim K. S., Morrison J. O. Amikacin + ceftazidime therapy of experimental right-sided Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1985;31(5):351–361. doi: 10.1159/000238359. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Norman D., Kim K. S. Efficacy of amikacin and ceftazidime in experimental aortic valve endocarditis due to Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1985 Dec;28(6):781–785. doi: 10.1128/aac.28.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Godfrey A. J., Schollardt T. Virulence of Pseudomonas aeruginosa strains with mechanisms of microbial persistence for beta-lactam and aminoglycoside antibiotics in a mouse infection model. Can J Microbiol. 1985 Apr;31(4):377–380. doi: 10.1139/m85-072. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hackbarth C. J., Drake T. A., Rusnak M. G., Sande M. A. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to beta-lactam antibiotics in vivo and in vitro. J Infect Dis. 1984 Jun;149(6):894–903. doi: 10.1093/infdis/149.6.894. [DOI] [PubMed] [Google Scholar]
- Choi C., Bayer A. S., Fujita N. K., Lam K., Guze L. B., Yoshikawa T. T. Therapy of experimental Pseudomonas endocarditis with high-dose amikacin and ticarcillin. Chemotherapy. 1983;29(4):303–312. doi: 10.1159/000238213. [DOI] [PubMed] [Google Scholar]
- Iida K., Koike M. Cell wall alterations of gram-negative bacteria by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Jan;5(1):95–97. doi: 10.1128/aac.5.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. D. Patterns and mechanisms of emergence of resistance to amikacin. J Infect Dis. 1977 Sep;136(3):449–452. doi: 10.1093/infdis/136.3.449. [DOI] [PubMed] [Google Scholar]
- Price K. E., Kresel P. A., Farchione L. A., Siskin S. B., Karpow S. A. Epidemiological studies of aminoglycoside resistance in the U.S.A. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):89–105. doi: 10.1093/jac/8.suppl_a.89. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):314–321. doi: 10.1093/clinids/5.2.314. [DOI] [PubMed] [Google Scholar]
- Shannon K., Phillips I. Mechanisms of resistance to aminoglycosides in clinical isolates. J Antimicrob Chemother. 1982 Feb;9(2):91–102. doi: 10.1093/jac/9.2.91. [DOI] [PubMed] [Google Scholar]
- Shekar R., Rice T. W., Zierdt C. H., Kallick C. A. Outbreak of endocarditis caused by Pseudomonas aeruginosa serotype O11 among pentazocine and tripelennamine abusers in Chicago. J Infect Dis. 1985 Feb;151(2):203–208. doi: 10.1093/infdis/151.2.203. [DOI] [PubMed] [Google Scholar]
- Waisbren S. J., Hurley D. J., Waisbren B. A. Morphological expressions of antibiotic synergism against Pseudomonas aeruginosa as observed by scanning electron microscopy. Antimicrob Agents Chemother. 1980 Dec;18(6):969–975. doi: 10.1128/aac.18.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]