Abstract
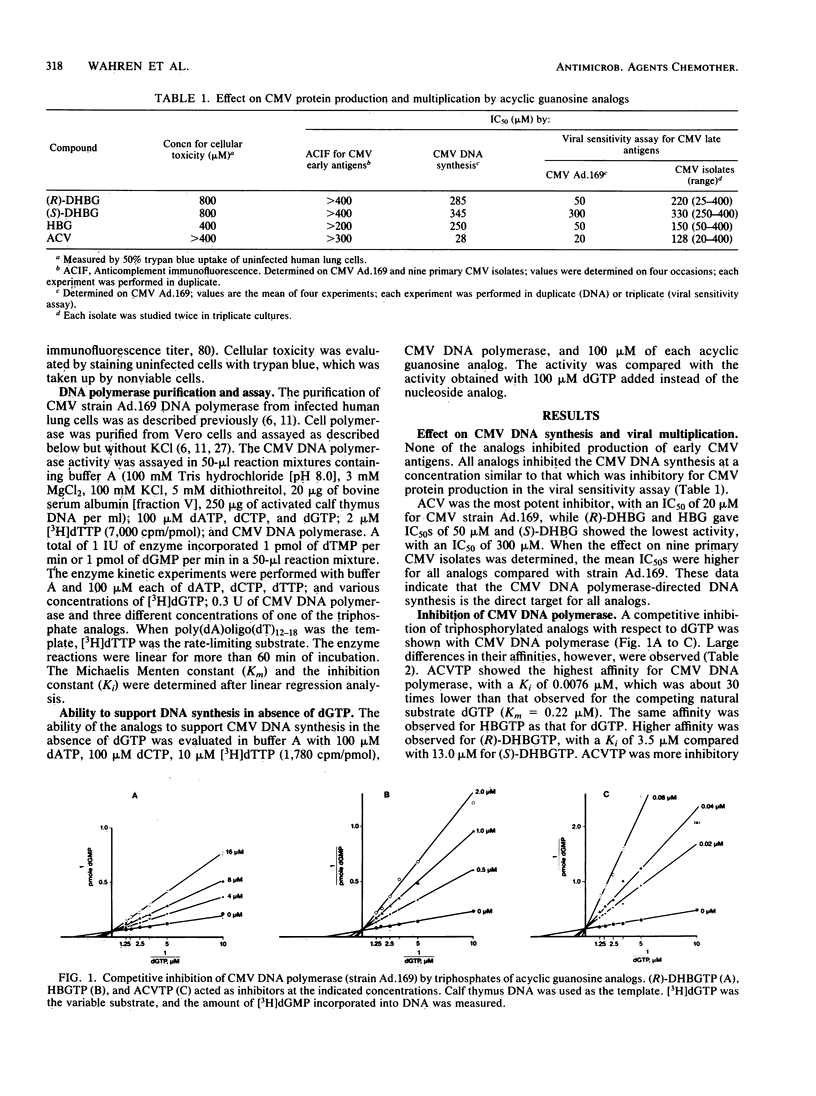
The acyclic guanosine analogs R- and S-enantiomers of 9-(3,4-dihydroxybutyl)guanine [(R)- and (S)-DHBG], 9-(4-hydroxybutyl)guanine (HBG), and 9-(2-hydroxyethoxymethyl)guanine (ACV) were examined for their effects on human cytomegalovirus (CMV) replication and on CMV DNA synthesis in cell culture as well as for their ability as triphosphates to interact with CMV DNA polymerase. Production of early CMV antigens was not affected. All analogs inhibited CMV DNA synthesis and late viral antigen synthesis. Primary CMV isolates were less susceptible to all tested analogs than was the laboratory strain CMV Ad.169. The triphosphate of ACV was the most potent inhibitor of CMV DNA polymerase, with an observed Ki of 0.0076 microM. The corresponding Ki values of the triphosphates of (R)-DHBG, (S)-DHBG, and HBG were 3.5, 13.0 and 0.23 microM, respectively. All triphosphates of the analogs given above inhibited CMV DNA polymerase in a competitive manner with respect to dGTP. The triphosphates of the analogs also inhibited reactions when the synthetic template poly(dC)oligo(dG)12-18 was used, whereas no inhibition was observed with poly(dA)oligo(dT)12-18. None of the triphosphate analogs supported DNA synthesis in the absence of dGTP, showing that no analog was an alternative substrate to dGTP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Walker R. T. Synthesis and antiviral properties of 5-vinylpyrimidine nucleoside analogs. Pharmacol Ther. 1984;26(1):1–44. doi: 10.1016/0163-7258(84)90049-4. [DOI] [PubMed] [Google Scholar]
- Ericson A. C., Larsson A., Aoki F. Y., Yisak W. A., Johansson N. G., Oberg B., Datema R. Antiherpes effects and pharmacokinetic properties of 9-(4-hydroxybutyl) guanine and the (R) and (S) enantiomers of 9-(3,4-dihydroxybutyl)guanine. Antimicrob Agents Chemother. 1985 May;27(5):753–759. doi: 10.1128/aac.27.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson B., Oberg B., Wahren B. Pyrophosphate analogues as inhibitors of DNA polymerases of cytomegalovirus, herpes simplex virus and cellular origin. Biochim Biophys Acta. 1982 Feb 26;696(2):115–123. doi: 10.1016/0167-4781(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadler H. Nucleic acid hybridization for measurement of effects of antiviral compounds on human cytomegalovirus DNA replication. Antimicrob Agents Chemother. 1983 Sep;24(3):370–374. doi: 10.1128/aac.24.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmenberg J., Wahren B., Oberg B. Influence of cells and virus multiplicity on the inhibition of herpesviruses with acycloguanosine. Intervirology. 1980;14(5-6):239–244. doi: 10.1159/000149192. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A. H., Harmenberg J. G., Wahren B. E. Influence of acyclovir and bucyclovir on nucleotide pools in cells infected with herpes simplex virus type 1. Antimicrob Agents Chemother. 1986 May;29(5):821–824. doi: 10.1128/aac.29.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Virus drug-resistance: mechanisms and consequences. Antiviral Res. 1984 Apr;4(1-2):1–42. doi: 10.1016/0166-3542(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Larsson A., Alenius S., Johansson N. G., Oberg B. Antiherpetic activity and mechanism of action of 9-(4-hydroxybutyl)guanine. Antiviral Res. 1983 Aug;3(2):77–86. doi: 10.1016/0166-3542(83)90028-1. [DOI] [PubMed] [Google Scholar]
- Larsson A., Oberg B., Alenius S., Hagberg C. E., Johansson N. G., Lindborg B., Stening G. 9-(3,4-dihydroxybutyl)guanine, a new inhibitor of herpesvirus multiplication. Antimicrob Agents Chemother. 1983 May;23(5):664–670. doi: 10.1128/aac.23.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Sundqvist A., Parnerud A. M. Inhibition of herpes simplex virus-induced DNA polymerases and cellular DNA polymerase alpha by triphosphates of acyclic guanosine analogs. Mol Pharmacol. 1986 Jun;29(6):614–621. [PubMed] [Google Scholar]
- Larsson A., Tao P. Z. Phosphorylation of four acyclic guanosine analogs by herpes simplex virus type 2 thymidine kinase. Antimicrob Agents Chemother. 1984 Apr;25(4):524–526. doi: 10.1128/aac.25.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Chiou J. F., Cheng Y. C., Huang E. S. Inhibition of cellular DNA polymerase alpha and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl)guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985 Mar;53(3):776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Johansson N. G. The relative merits and drawbacks of new nucleoside analogues with clinical potential. J Antimicrob Chemother. 1984 Aug;14 (Suppl A):5–26. doi: 10.1093/jac/14.suppl_a.5. [DOI] [PubMed] [Google Scholar]
- Ruth J. L., Cheng Y. C. Nucleoside analogues with clinical potential in antivirus chemotherapy. The effect of several thymidine and 2'-deoxycytidine analogue 5'-triphosphates on purified human (alpha, beta) and herpes simplex virus (types 1, 2) DNA polymerases. Mol Pharmacol. 1981 Sep;20(2):415–422. [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Sundqvist V. A., Wahren B. An interchangeable ELISA for cytomegalovirus antigen and antibody. J Virol Methods. 1981 Apr;2(5):301–312. doi: 10.1016/0166-0934(81)90029-x. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Livelli T. J., Perry H. C., Crumpacker C. S., Field A. K. Effects of the nucleoside analog 2'-nor-2'-deoxyguanosine on human cytomegalovirus replication. Antimicrob Agents Chemother. 1984 Feb;25(2):247–252. doi: 10.1128/aac.25.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B., Harmenberg J., Sundqvist V. A., Levén B., Sköldenberg B. A novel method for determining the sensitivity of herpes simplex virus to antiviral compounds. J Virol Methods. 1983 Mar;6(3):141–149. doi: 10.1016/0166-0934(83)90026-5. [DOI] [PubMed] [Google Scholar]
- Wahren B., Oberg B. Inhibition of cytomegalovirus late antigens by phosphonoformate. Intervirology. 1980;12(6):335–339. doi: 10.1159/000149093. [DOI] [PubMed] [Google Scholar]
- Wahren B., Rudén U., Gadler H., Oberg B., Eriksson B. Activity of the cytomegalovirus genome in the presence of PPi analogs. J Virol. 1985 Dec;56(3):996–1001. doi: 10.1128/jvi.56.3.996-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B., Wahren P., Harmenberg J., Sundqvist V. A. Computer-based virus sensitivity assay and neutralization method. Applications for herpesviruses. J Virol Methods. 1983 May;6(5):271–282. doi: 10.1016/0166-0934(83)90042-3. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]


