Abstract
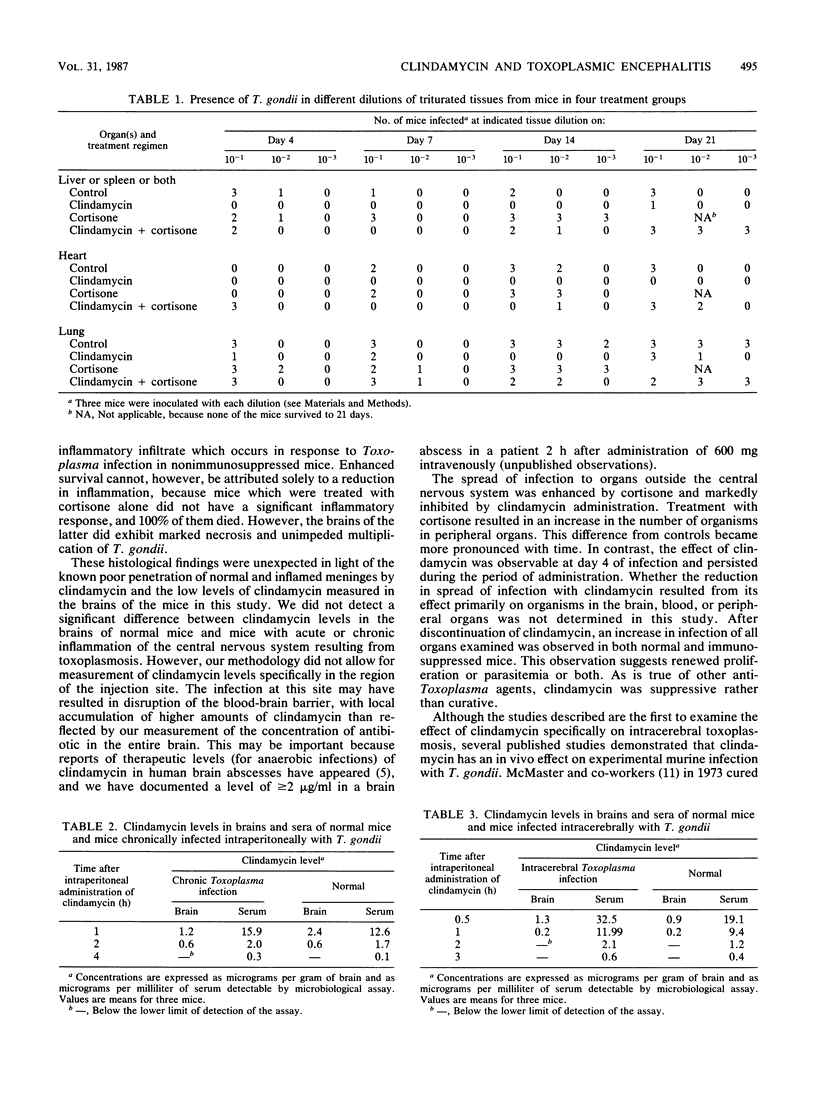
We investigated the efficacy of clindamycin in a murine model of toxoplasmic encephalitis using direct intracerebral inoculation. Clindamycin reduced mortality from 40% in normal mice and 100% in cortisone-treated mice to 0% in both groups. Although we were unable to document appreciable levels of clindamycin in the brains of infected mice, the histological features of cerebral infection were markedly altered. The formation of large numbers of cysts and the intense inflammatory response seen in the brains of normal mice and the unchecked infection and tissue necrosis in the brains of cortisone-treated mice were absent in the brains of clindamycin-treated mice. Enumeration of cysts in the brains of mice 10 weeks after infection revealed a significantly lower number in the clindamycin-treated mice. Spread of infection to other organs was also decreased during clindamycin administration. These observations suggest that clindamycin may have a role in the therapy of toxoplasmic encephalitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Remington J. S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother. 1974 Jun;5(6):647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley F. K., Jenkins K. A. Immunohistological study of the anatomic relationship of toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981 Mar;31(3):1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley F. K., Remington J. S. Nonspecific inhibition of tumor growth in the central nervous system: observations of intracerebral ependymoblastoma in mice with chronic Toxoplasma infection. J Natl Cancer Inst. 1977 Sep;59(3):963–973. doi: 10.1093/jnci/59.3.963. [DOI] [PubMed] [Google Scholar]
- DeHaan R. M., Metzler C. M., Schellenberg D., Vandenbosch W. D. Pharmacokinetic studies of clindamycin phosphate. J Clin Pharmacol. 1973 May-Jun;13(5):190–209. doi: 10.1002/j.1552-4604.1973.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Handman E., Chester P. M., Remington J. S. Delayed hypersensitivity to Toxoplasma and unrelated antigens in Toxoplasma-infected mice: induction and elicitation of delayed-type hypersensitivity by antigen-pulsed macrophages. Infect Immun. 1980 May;28(2):524–531. doi: 10.1128/iai.28.2.524-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster P. R., Powers K. G., Finerty J. F., Lunde M. N. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am J Trop Med Hyg. 1973 Jan;22(1):14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- Milligan S. A., Katz M. S., Craven P. C., Strandberg D. A., Russell I. J., Becker R. A. Toxoplasmosis presenting as panhypopituitarism in a patient with the acquired immune deficiency syndrome. Am J Med. 1984 Oct;77(4):760–764. doi: 10.1016/0002-9343(84)90381-4. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Thiermann E., Apt W., Atias A., Lorca M., Olguín J. A comparative study of some combined treatment regimens in acute toxoplasmosis in mice. Am J Trop Med Hyg. 1978 Jul;27(4):747–750. doi: 10.4269/ajtmh.1978.27.747. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Novak E., Patel N. C., Chidester C. G., Lummis W. L. Absorption, excretion and half-life of clinimycin in normal adult males. Am J Med Sci. 1968 Jul;256(1):25–37. doi: 10.1097/00000441-196807000-00004. [DOI] [PubMed] [Google Scholar]







