Abstract
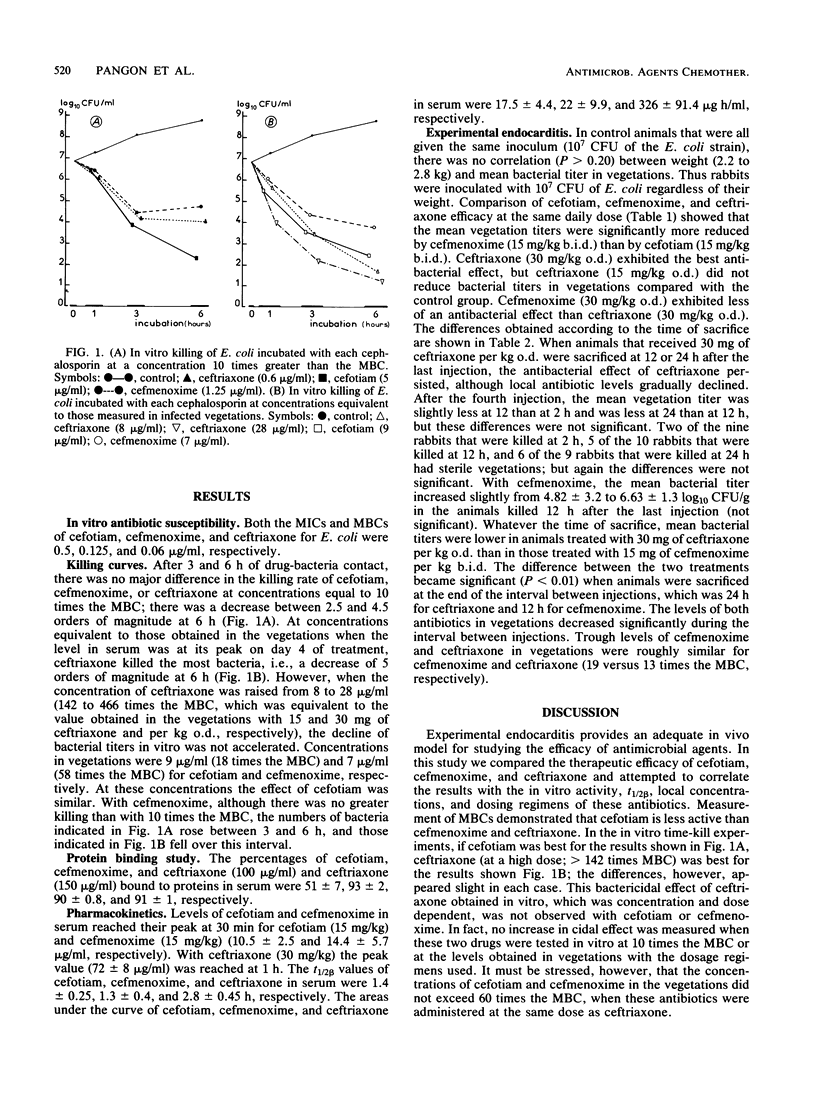
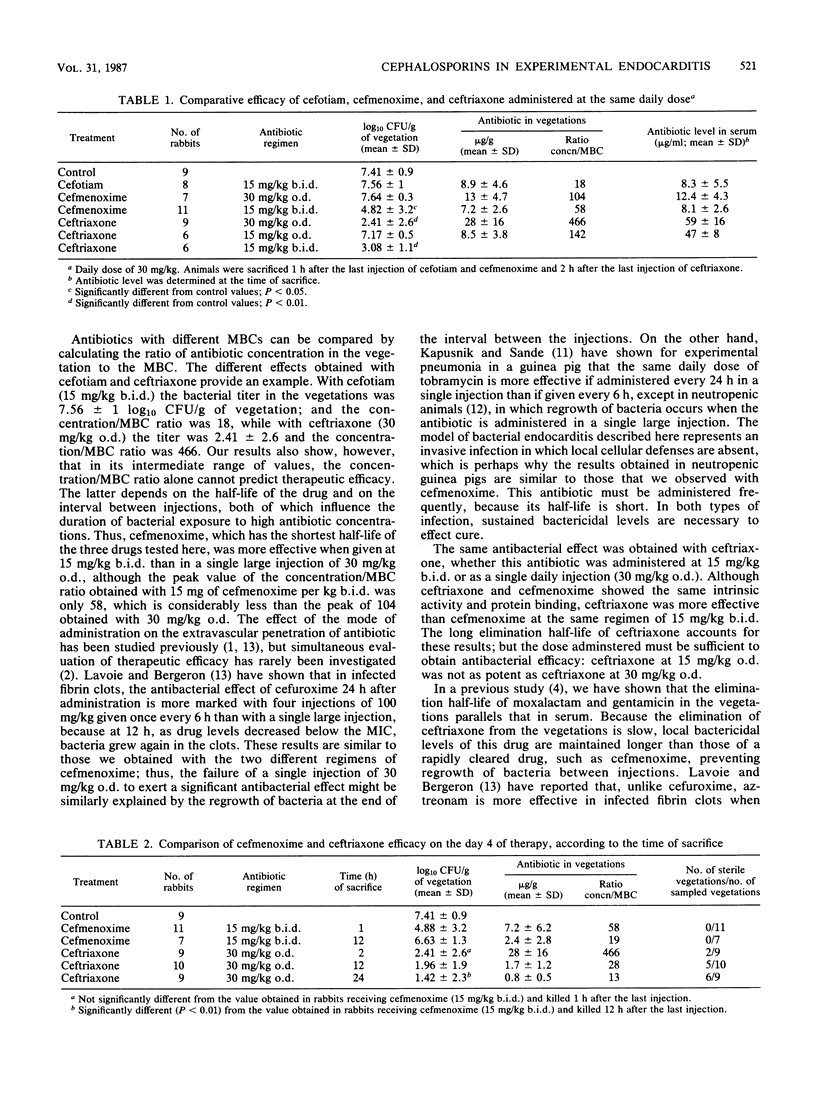
To determine the influence of in vitro activity, pharmacokinetic properties, and therapeutic regimen on the antibacterial effect in vivo, we compared three cephalosporins, cefotiam, cefmenoxime, and ceftriaxone, in a rabbit model of experimental Escherichia coli endocarditis after 4 days of treatment. The MBCs of cefotiam, cefmenoxime, and ceftriaxone for the E. coli strain were 0.5, 0.125, and 0.06 microgram/ml, respectively. Killing curves at 10 times the MBC were similar for the three cephalosporins. In serum, the elimination half-life of ceftriaxone was twice as much as the elimination half-life of cefotiam or cefmenoxime (2.8 +/- 0.45 versus 1.4 +/- 0.25 or 1.3 +/- 0.4 h, respectively). Ceftriaxone was much more effective than cefotiam. The bacterial titer in the vegetations (log10 CFU per gram of vegetation) was 7.56 +/- 1 with cefotiam and 2.41 +/- 2.6 with ceftriaxone, as their concentrations were 18 and 466 times higher, respectively, than their MBCs. Although ceftriaxone and cefmenoxime exhibited a similar rate of killing and percentage of protein binding, ceftriaxone was more effective than cefmenoxime at the same regimen of 15 mg/kg twice a day (3.08 +/- 1.1 versus 4.82 +/- 3.2 log10 CFU/g of vegetation). When antibiotic was given as a single daily injection of 30 mg/kg, the antibacterial effect persisted for ceftriaxone, but not for cefmenoxime. The longer elimination half-life and the higher local concentration/MBC ratio of ceftriaxone explained these results. The bacterial titer measured 24 h after the fourth injection of 30 mg of ceftriaxone per kg confirmed that this regimen prevented regrowth of bacteria. These results suggest that the local antibiotic level/MBC ratio roughly correlated with the antibacterial effect and could represent an adequate basis to explain the differences observed between the drugs in vivo. They also demonstrate that, provided that the dose is sufficient, a long-acting broad-spectrum cephalosporin may be effective in severe gram-negative infections, even when given at relatively long dosing intervals, in contrast with a rapidly cleared drug with the same intrinsic activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron M. G., Beauchamp D., Poirier A., Bastille A. Continuous vs. intermittent administration of antimicrobial agents: tissue penetration and efficacy in vivo. Rev Infect Dis. 1981 Jan-Feb;3(1):84–97. doi: 10.1093/clinids/3.1.84. [DOI] [PubMed] [Google Scholar]
- Bouvet A., Cremieux A. C., Contrepois A., Vallois J. M., Lamesch C., Carbon C. Comparison of penicillin and vancomycin, individually and in combination with gentamicin and amikacin, in the treatment of experimental endocarditis induced by nutritionally variant streptococci. Antimicrob Agents Chemother. 1985 Nov;28(5):607–611. doi: 10.1128/aac.28.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrepois A., Vallois J. M., Garaud J. J., Pangon B., Mohler J., Meulemans A., Carbon C. Kinetics and bactericidal effect of gentamicin and latamoxef (moxalactam) in experimental Escherichia coli endocarditis. J Antimicrob Chemother. 1986 Feb;17(2):227–237. doi: 10.1093/jac/17.2.227. [DOI] [PubMed] [Google Scholar]
- Daschner F. D., Hemmer K. A., Offermann P., Slanicka J. Pharmacokinetics of cefotiam in normal humans. Antimicrob Agents Chemother. 1982 Dec;22(6):958–960. doi: 10.1128/aac.22.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decazes J. M., Ernst J. D., Sande M. A. Correlation of in vitro time-kill curves and kinetics of bacterial killing in cerebrospinal fluid during ceftriaxone therapy of experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1983 Oct;24(4):463–467. doi: 10.1128/aac.24.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Pelletier L. L., Petersdorf R. G. Chemotherapy of experimental streptococcal endocarditis. II. Synergism between penicillin and streptomycin against penicillin-sensitive streptococci. J Clin Invest. 1974 Mar;53(3):829–833. doi: 10.1172/JCI107622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Granneman G. R., Sennello L. T., Steinberg F. J., Sonders R. C. Intramuscular and intravenous pharmacokinetics of cefmenoxime, a new broad-spectrum cephalosporin, in healthy subjects. Antimicrob Agents Chemother. 1982 Jan;21(1):141–145. doi: 10.1128/aac.21.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusnik J. E., Sande M. A. Novel approaches for the use of aminoglycosides: the value of experimental models. J Antimicrob Chemother. 1986 Mar;17 (Suppl A):7–10. doi: 10.1093/jac/17.suppl_a.7. [DOI] [PubMed] [Google Scholar]
- Lavoie G. Y., Bergeron M. G. Influence of four modes of administration on penetration of aztreonam, cefuroxime, and ampicillin into interstitial fluid and fibrin clots and on in vivo efficacy against Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Sep;28(3):404–412. doi: 10.1128/aac.28.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. S., Komarmy L. New method for detecting in vitro inactivation of penicillins by Haemophilus influenzae and Staphlycoccus aureus. Antimicrob Agents Chemother. 1976 Sep;10(3):564–566. doi: 10.1128/aac.10.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Johnson M. L. Antimicrobial therapy of experimental endocarditis caused by Staphylococcus aureus. J Infect Dis. 1975 Apr;131(4):367–375. doi: 10.1093/infdis/131.4.367. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Sande M. A. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Invest. 1983 Mar;71(3):411–419. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Reller L. B. Correlation of serum bactericidal activity with antimicrobial agent level and minimal bactericidal concentration. J Infect Dis. 1982 Feb;145(2):160–168. doi: 10.1093/infdis/145.2.160. [DOI] [PubMed] [Google Scholar]



