Abstract
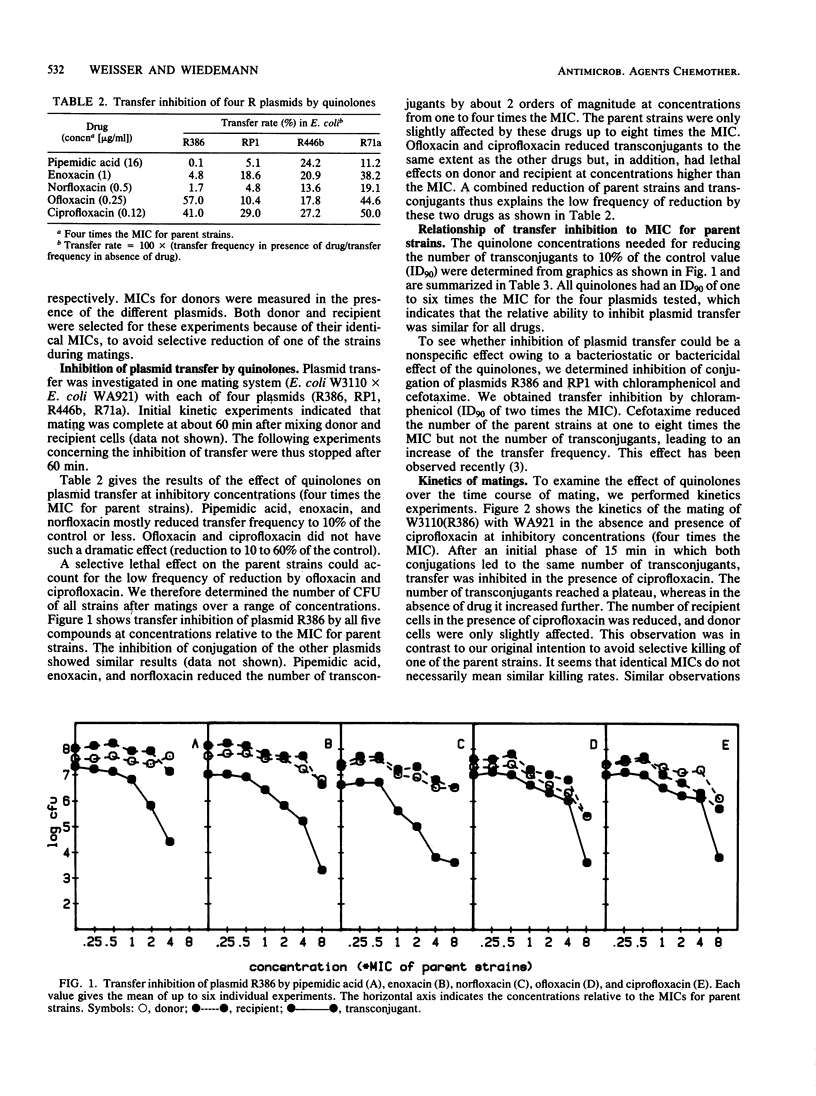
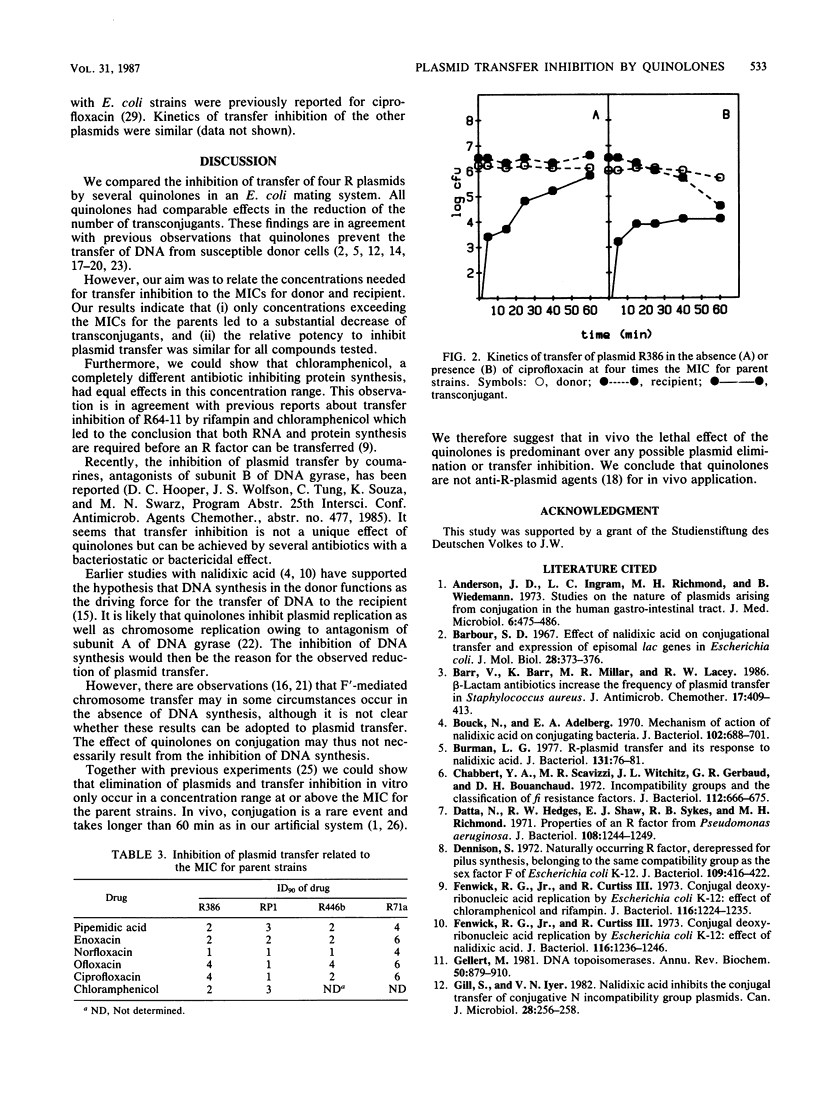
Inhibition of transfer of four conjugative R plasmids by ciprofloxacin, enoxacin, norfloxacin, ofloxacin, and pipemidic acid was investigated in an Escherichia coli mating system. The absolute concentrations needed for inhibition of conjugation varied from 0.12 microgram/ml for ciprofloxacin to 16 micrograms/ml for pipemidic acid, but the relationship to the MICs for the parent strains was identical for all substrates. Concentrations for a 90% reduction of transconjugants were in the range of one to six times the MIC for the parent strains, which also had lethal effects on donors and recipients. A similar effect on conjugation was found with chloramphenicol. These observations question the specificity of transfer inhibition by quinolones and cast doubt on the clinical importance of such an effect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Ingram L. C., Richmond M. H., Wiedemann B. Studies on the nature of plasmids arising from conjugation in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):475–486. doi: 10.1099/00222615-6-4-475. [DOI] [PubMed] [Google Scholar]
- Barbour S. D. Effect of nalidixic acid on conjugational transfer and expression of episomal lac genes in Escherichia coli K12. J Mol Biol. 1967 Sep 14;28(2):373–376. doi: 10.1016/s0022-2836(67)80016-0. [DOI] [PubMed] [Google Scholar]
- Barr V., Barr K., Millar M. R., Lacey R. W. Beta-lactam antibiotics increase the frequency of plasmid transfer in Staphylococcus aureus. J Antimicrob Chemother. 1986 Apr;17(4):409–413. doi: 10.1093/jac/17.4.409. [DOI] [PubMed] [Google Scholar]
- Bouck N., Adelberg E. A. Mechanism of action of nalidixic acid on conjugating bacteria. J Bacteriol. 1970 Jun;102(3):688–701. doi: 10.1128/jb.102.3.688-701.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G. R-plasmid transfer and its response to nalidixic acid. J Bacteriol. 1977 Jul;131(1):76–81. doi: 10.1128/jb.131.1.76-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison S. Naturally occurring R factor, derepressed for pilus synthesis, belonging to the same compatibility group as the sex factor F of Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):416–422. doi: 10.1128/jb.109.1.416-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: effect of nalidixic acid. J Bacteriol. 1973 Dec;116(3):1236–1246. doi: 10.1128/jb.116.3.1236-1246.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Excherichia coli K-12: effect of chloramphenicol and rifampin. J Bacteriol. 1973 Dec;116(3):1224–1235. doi: 10.1128/jb.116.3.1224-1235.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gill S., Iyer V. N. Nalidixic acid inhibits the conjugal transfer of conjugative N incompatibility group plasmids. Can J Microbiol. 1982 Feb;28(2):256–258. doi: 10.1139/m82-034. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- Michel-Briand Y., Laporte J. M., Bassignot A. Inhibition du transfert de plasmides chez Escherichia coli, par l'acide oxolinique. C R Seances Acad Sci III. 1983;296(21):989–994. [PubMed] [Google Scholar]
- Nakamura S., Inoue S., Shimizu M., Iyobe S., Mitsuhashi S. Inhibition of conjugal transfer of R plasmids by pipemidic acid and related compounds. Antimicrob Agents Chemother. 1976 Nov;10(5):779–785. doi: 10.1128/aac.10.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva B., Selan L., Ravagnan G., Renzini G. Inhibition of conjugal transfer by new quinolinic compounds. Chemioterapia. 1985 Jun;4(3):199–201. [PubMed] [Google Scholar]
- Sarathy P. V., Siddiqi O. DNA synthesis during bacterial conjugation. II. Is DNA replication in the Hfr obligatory for chromosome transfer? J Mol Biol. 1973 Aug 15;78(3):443–451. doi: 10.1016/0022-2836(73)90467-1. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuye A. Effect of cinoxacin on the conjugal transfer of R-plasmids in Escherichia coli. J Pharm Belg. 1980 Nov-Dec;62(6):451–455. [PubMed] [Google Scholar]
- Weisser J., Wiedemann B. Elimination of plasmids by new 4-quinolones. Antimicrob Agents Chemother. 1985 Nov;28(5):700–702. doi: 10.1128/aac.28.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann B. Die Ubertragung extrachromosomaler Resistenzfaktoren in der Darmflora und ihre Hemmung. Zentralbl Bakteriol Orig A. 1972 May;220(1):106–123. [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Zeiler H. J. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1985 Oct;28(4):524–527. doi: 10.1128/aac.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]



