Abstract
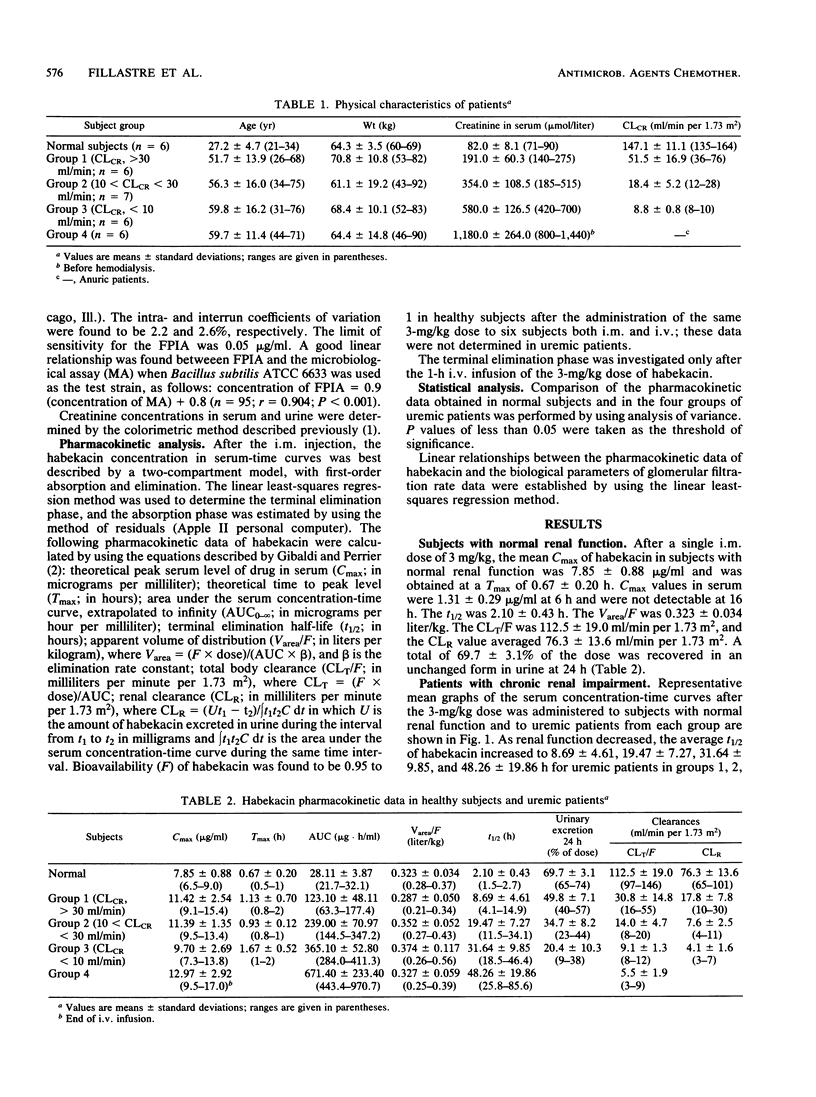
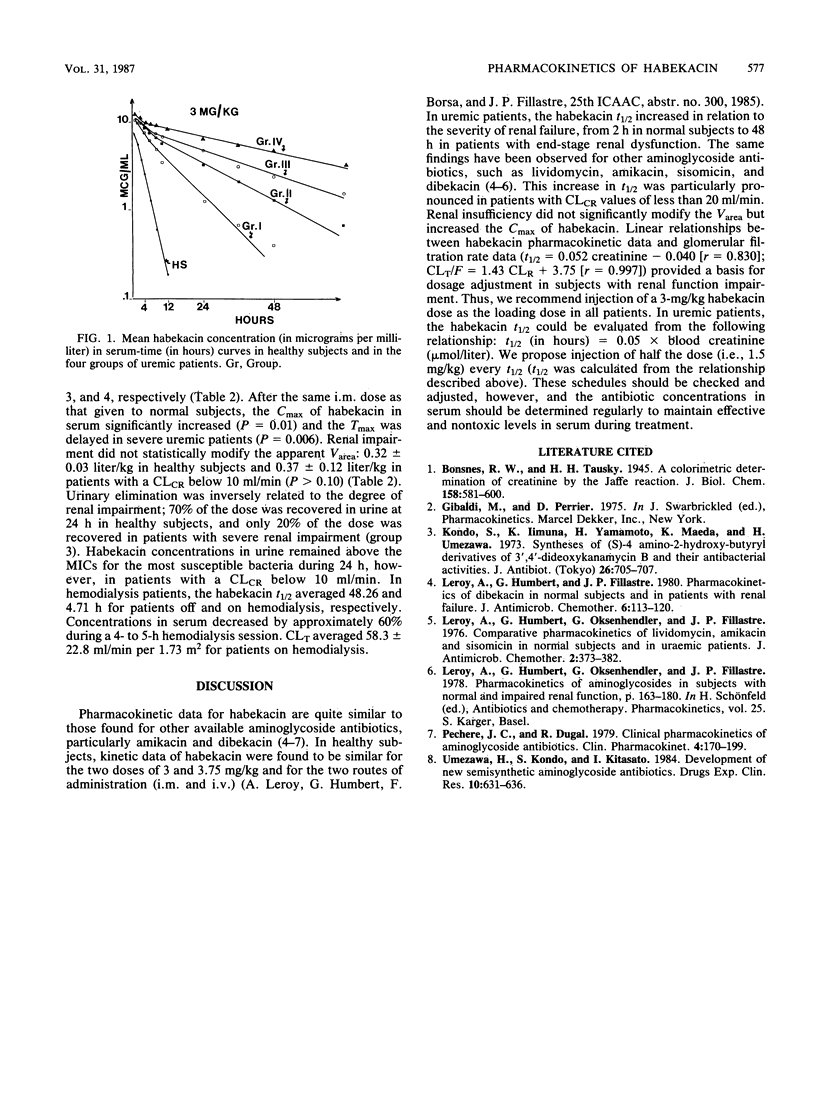
The pharmacokinetics of habekacin, a new semisynthetic aminoglycoside antibiotic, were investigated in six healthy subjects and 25 uremic patients (six of whom were on hemodialysis) after administration of a single 3-mg/kg dose. Six healthy subjects received the 3-mg/kg dose both intramuscularly (i.m.) and intravenously (i.v.) (1-h infusion). Uremic patients were given the 3-mg/kg dose as an i.m. injection, except for the hemodialysis patients, who received the dose as a 1-h i.v. infusion. After the i.m. injection, the peak concentrations in serum were higher and the times to peak levels were longer in patients with renal impairment than in healthy subjects. The elimination half-life in serum increased in relation to the degree of renal impairment, from 2 h in normal subjects to 32 h in patients with creatinine clearances of less than 10 ml/min. Renal impairment did not significantly modify the apparent volume of distribution. After the same 3-mg/kg dose as a 1-h i.v. infusion in six hemodialysis patients, the elimination half-life averaged 48 and 5 h off and on a 4- to 5-h hemodialysis session, respectively. The habekacin pharmacokinetic data appeared to be similar to those of the other available aminoglycoside antibiotics.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kondo S., Iinuma K., Yamamoto H., Ikeda Y., Maeda K. Letter: Synthesis of (S)-4-amino-2-hydroxybutyryl derivatives of 3',4'-dideoxykanamycin B and their antibacterial activities. J Antibiot (Tokyo) 1973 Nov;26(11):705–707. doi: 10.7164/antibiotics.26.705. [DOI] [PubMed] [Google Scholar]
- Leroy A., Humbert G., Fillastre J. P. Pharmacokinetics of dibekacin in normal subjects and in patients with renal failure. J Antimicrob Chemother. 1980 Jan;6(1):113–120. doi: 10.1093/jac/6.1.113. [DOI] [PubMed] [Google Scholar]
- Leroy A., Humbert G., Oksenhendler G., Fillastre J. P. Comparative pharmacokinetics of lividomycin, amikacin and sisomicin in normal subjects and in uraemic patients. J Antimicrob Chemother. 1976 Dec;2(4):373–381. doi: 10.1093/jac/2.4.373. [DOI] [PubMed] [Google Scholar]
- Leroy A., Humbert G., Oksenhendler G., Fillastre J. P. Pharmacokinetics of aminoglycosides in subjects with normal and impaired renal function. Antibiot Chemother (1971) 1978;25:163–180. doi: 10.1159/000401061. [DOI] [PubMed] [Google Scholar]
- Pechere J. C., Dugal R. Clinical pharmacokinetics of aminoglycoside antibiotics. Clin Pharmacokinet. 1979 May-Jun;4(3):170–199. doi: 10.2165/00003088-197904030-00002. [DOI] [PubMed] [Google Scholar]


