Abstract
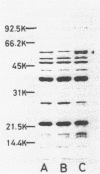
Two genetically distinct classes of norfloxacin-resistant Pseudomonas aeruginosa PAO4009 mutants were isolated spontaneously. Two norfloxacin resistance genes, nfxA and nfxB, were mapped hex-9001 and leu-9005 and between pro-9031 and ilv-9023, respectively, on the P. aeruginosa PAO chromosome. The nfxA gene was shown to be an allele of nalA by transductional analysis with bacteriophage F116L. The nfxB mutant showed a 16-fold increase in resistance to norfloxacin and a slight increase in resistance to nalidixic acid. The nfxB mutant was unique in that it showed hypersusceptibility to beta-lactam and aminoglycoside antibiotics. This mutant had about a threefold-lower rate of norfloxacin uptake than that of the wild-type strain or nfxA mutant. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of outer membrane proteins demonstrated the appearance of a 54,000-dalton protein in the nfxB mutant. These findings suggested that the norfloxacin resistance mechanism in the nfxB mutant might be an alteration in outer membrane permeability to norfloxacin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G. Apparent absence of transferable resistance to nalidixic acid in pathogenic Gram-negative bacteria. J Antimicrob Chemother. 1977 Sep;3(5):509–516. doi: 10.1093/jac/3.5.509. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Govan J. R. Chromosomal loci associated with antibiotic hypersensitivity in pulmonary isolates of Pseudomonas aeruginosa. J Gen Microbiol. 1984 Apr;130(4):825–834. doi: 10.1099/00221287-130-4-825. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Hosaka M., Oomori Y., Niwata Y., Suzue S., Irikura T. In vitro and in vivo antibacterial activity of AM-833, a new quinolone derivative. Antimicrob Agents Chemother. 1986 Jun;29(6):1059–1066. doi: 10.1128/aac.29.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Ito A., Abe Y., Suzue S., Irikura T., Inoue M., Mitsuhashi S. Comparative activities of AM-715 and pipemidic and nalidixic acids against experimentally induced systemic and urinary tract infections. Antimicrob Agents Chemother. 1981 Jan;19(1):188–189. doi: 10.1128/aac.19.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrebenda J., Heleszko H., Brzostek K., Bielecki J. Mutation affecting resistance of Escherichia coli K12 to nalidixic acid. J Gen Microbiol. 1985 Sep;131(9):2285–2292. doi: 10.1099/00221287-131-9-2285. [DOI] [PubMed] [Google Scholar]
- Inoue S., Ohue T., Yamagishi J., Nakamura S., Shimizu M. Mode of incomplete cross-resistance among pipemidic, piromidic, and nalidixic acids. Antimicrob Agents Chemother. 1978 Aug;14(2):240–245. doi: 10.1128/aac.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Govan J. W., Fyfe J. A., Costerton J. W. Heterogeneity of antibiotic resistance in mucoid isolates of Pseudomonas aeruginosa obtained from cystic fibrosis patients: role of outer membrane proteins. Antimicrob Agents Chemother. 1981 Jun;19(6):1056–1063. doi: 10.1128/aac.19.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V. A novel transducing phage. Its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1972;114(2):134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Ohta S., Kobayashi R., Terawaki Y. Chromosomal location of genes participating in the degradation of purines in Pseudomonas aeruginosa. Mol Gen Genet. 1978 Nov 29;167(2):165–176. doi: 10.1007/BF00266910. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T. FP5 factor, an undescribed sex factor of Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Sep;17(5):409–417. doi: 10.1111/j.1348-0421.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Scurlock T. R. DNA gyrase (Topoisomerase II) from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1983 Jan 27;110(2):694–700. doi: 10.1016/0006-291x(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Mills B. J., Holloway B. W. Mutants of Pseudomonas aeruginosa that show specific hypersensitivity to aminoglycosides. Antimicrob Agents Chemother. 1976 Sep;10(3):411–416. doi: 10.1128/aac.10.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okii M., Iyobe S., Mitsuhashi S. Mapping of the gene specifying aminoglycoside 3'-phosphotransferase II on the Pseudomonas aeruginosa chromosome. J Bacteriol. 1983 Aug;155(2):643–649. doi: 10.1128/jb.155.2.643-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. Purification and properties of an S-type pyocin, pyocin AP41. J Bacteriol. 1981 May;146(2):733–739. doi: 10.1128/jb.146.2.733-739.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer B. G., Legakis N. J. Pseudomonas aeruginosa: evidence for the involvement of lipopolysaccharide in determining outer membrane permeability to carbenicillin and gentamicin. J Infect Dis. 1985 Aug;152(2):351–355. doi: 10.1093/infdis/152.2.351. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]