Abstract
Apoptosis is an innate immune response to viral infection that limits viral replication. However, the mechanisms by which cells detect viral infection and activate apoptosis are not completely understood. We now show that during Coxsackievirus infection, the viral protease 3Cpro cleaves inhibitor of κBα (IκBα). A proteolytic fragment of IκBα then forms a stable complex with NF-κB, translocates to the nucleus, and inhibits NF-κB transactivation, increasing apoptosis and decreasing viral replication. In contrast, cells with reduced IκBα expression are more susceptible to viral infection, with less apoptosis and more viral replication. IκBα thus acts as a sensor of viral infection. Cleavage of host proteins by pathogen proteases is a novel mechanism by which the host recognizes and responds to viral infection.
Keywords: Coxsackievirus, nitric oxide, Picornavirus
Apoptosis is an innate immune response to viral infection (1, 2). Virus infection can indirectly induce apoptosis of the host cell by stimulating immune effector cells, which in turn activate apoptosis of infected cells. Virus infection can also directly trigger apoptosis of host cells, but the mechanisms by which infected cells sense viral infection and activate programmed cell death are less well understood.
The family of NF-κB transcription factors not only activates proinflammatory pathways in innate and adaptive immunity, but also plays a critical role in preventing apoptosis (3–7). Human NF-κB is composed of a homodimer or heterodimer of proteins than belong to the rel family, including p65, p50, c-rel, and rel-B. NF-κB proteins are normally retained in the cytoplasm by members of the inhibitor of κB (IκB) family (4–6). However, a variety of agonists can stimulate cell signaling pathways that release NF-κB factors. NF-κB proteins then translocate into the nucleus and activate transcription of proinflammatory genes and also transcription of antiapoptotic genes, including cIAP1, XIAP, c-FLIP, Bcl-2, and Bcl-XL. NF-κB activation can prevent apoptosis that would otherwise be induced by cytotoxic proteins such as TNF-α. Activation of apoptosis by TNF-α depends in part on deactivation of NF-κB signaling (8–10). Inhibition of the antiapoptotic effects of NF-κB may be beneficial to the host during viral infection, decreasing viral replication and increasing host survival.
We hypothesized that components of the apoptotic pathway serve as intracellular sensors for viral infection. Host substrates in the apoptotic pathway that are cleaved by viral proteases might activate apoptosis, thereby limiting viral replication. We now show that the Coxsackievirus protease 3Cpro cleaves IκBα, generating an IκBα fragment that represses NF-κB signaling, blocks the antiapoptotic effects of NF-κB, and decreases viral replication.
Results
Coxsackievirus B3 (CVB3) Induces Apoptosis.
CVB3 infection of HeLa cells causes apoptosis, as detected by DAPI staining of nuclear chromatin after infection, nucleosomal fragments, and poly(ADP-ribose) polymerase (PARP) cleavage (Fig. 1). Because NF-κB signaling regulates apoptosis, we next explored the effect of Coxsackievirus infection upon nuclear translocation of NF-κB. HeLa cells were infected with CVB3 for 0–240 min or treated with TNF-α for 30 min as a control, and nuclear localization of the NF-κB p65 subunit was assayed by immunoblotting and immunohistochemistry. After infection with CVB3, NF-κB appears in the nucleus, although more slowly than after TNF-α treatment. We therefore sought to characterize more fully the effects of viral infection upon NF-κB signal transduction.
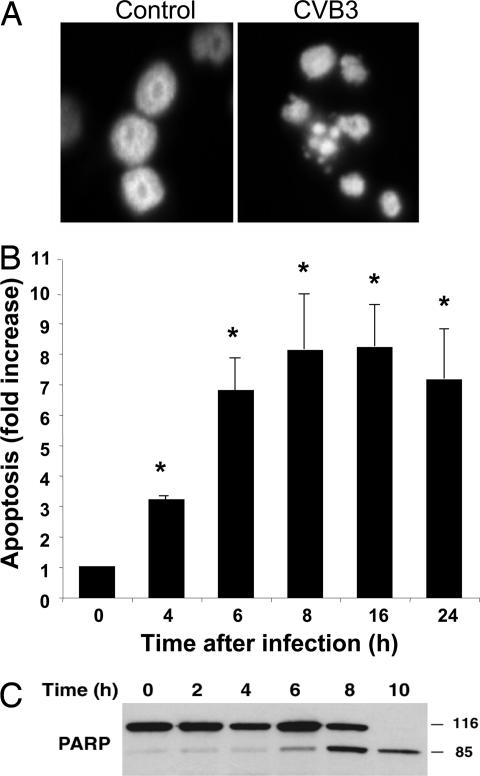
Fig. 1.
CVB3 induces apoptosis in HeLa cells. HeLa cells were infected with CVB3 at a multiplicity of infection of 1, and apoptosis was measured by DAPI staining of nuclei (A), ELISA for the detection of a histone–DNA complex (n = 3 ± SD; ∗, P < 0.05) (B), and Western blot of PARP in cell lysates (C). (Magnification: A, ×120.)
CVB3 Infection Does Not Activate NF-κB.
We then examined the effect of CVB3 infection on NF-κB activation. HeLa cells were infected with CVB3 or treated 30 min with TNF-α. Nuclear extracts were isolated and subjected to EMSA with radiolabeled NF-κB oligonucleotide DNA binding domain sequence (Fig. 2C Left). In addition, HeLa cells were transiently transfected with a κB-luciferase reporter construct, and then treated with TNF-α or CVB3 (Fig. 2C Right). TNF-α significantly increases NF-κB DNA binding and transactivation, as expected (Fig. 2C Left, lane 2; also Fig. 2C Right). However, CVB3 infection neither increases NF-κB DNA binding (Fig. 2C Left, lanes 3–5) nor transactivation of the reporter gene at any time after infection (Fig. 2C Right). Not only does CVB3 infection fail to activate a κB reporter construct, but in addition CVB3 inhibits TNF-α-mediated NF-κB transactivation (Fig. 7, which is published as supporting information on the PNAS web site). (These experiments included an internal control, cotransfection with a vector expressing Renilla luciferase driven by a CMV promoter.) Taken together, these data suggest a paradox: although CVB3 infection leads to nuclear localization of NF-κB, CVB3 inhibits NF-κB binding activity and transactivation.
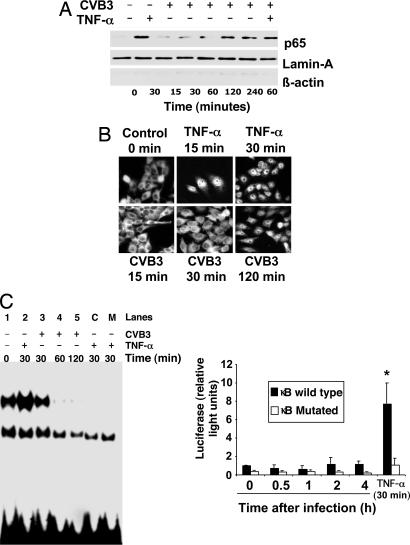
Fig. 2.
NF-κB activation is impaired during viral infection. (A) HeLa cells were treated with TNF-α or infected with CVB3, and nuclear extracts were harvested and immunoblotted with antibody to p65, lamin-A, and β-actin. (B) HeLa cells treated with TNF-α or infected with CVB3 for 0–120 min were analyzed by immunocytofluorescence with antibody to p65. CVB3 induces delayed translocation of NF-κB into the nucleus after 120 min. (C) (Left) Nuclear extracts were isolated from HeLa cells infected with CVB3 at different time points or treated with TNF-α as before and subjected to EMSA assay with radiolabeled oligonucleotides containing the NF-κB DNA binding domain sequence. In addition, extracts from TNF-α treated cells were incubated with a 100-fold of unlabeled oligonucleotide (lane C) and with a scrambled oligonucleotide for the NF-κB binding sequence (lane M). (Right) HeLa cells were transfected with a WT or mutant κB element-luciferase reporter construct, and then infected with CVB3 for 0–4 h or treated with TNF-α for 30 min, and luciferase expression was measured by a luminometer (n = 3 ± SD; ∗, P < 0.05). Although NF-κB enters the nucleus, the NF-κB reporter gene is not expressed. (Magnification: B, ×40.)
CVB3 Infection Alters IκBα Location.
To study how CVB3 inhibits NF-κB binding and transactivation, we localized IκBα in HeLa cells. CVB3 infection does not change steady-state levels of IκBα (Fig. 3A). However, we detected an additional 30-kDa IκBα fragment in infected HeLa cells, total cell extracts (Fig. 3A Upper), or nuclear extracts (Fig. 3A Lower). The fragment of IκBα within the nucleus is bound to NF-κB, because immunoprecipitants prepared from nuclei of infected cells with antibody to the NF-κB p65 subunit contain the amino-terminal IκBα fragment (Fig. 3B). These findings suggest that CVB3 infection leads to cleavage of IκBα. Furthermore, a fragment of IκBα enters the nucleus where it interacts with NF-κB.
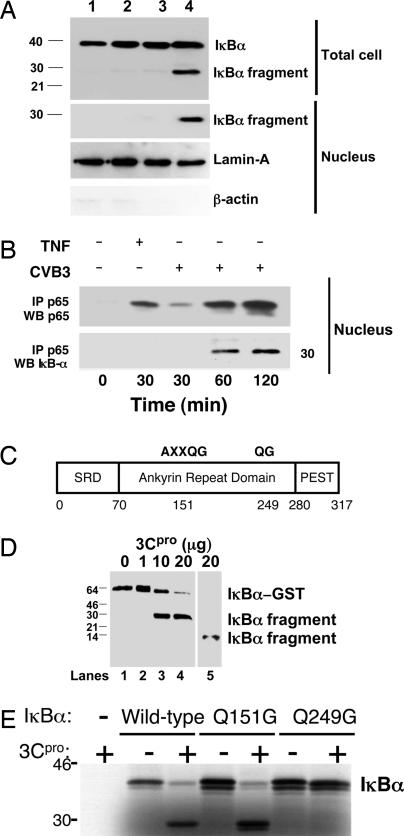
Fig. 3.
The CVB3 viral protease 3Cpro cleaves IκBα. (A) HeLa cells were treated with media alone (lane 1) or CVB3 for 15 min (lane 2), 30 min (lane 3), or 120 min (lane 4). Total cell lysates (Upper) and nuclear extracts (Lower) were immunoblotted with antibody to IκBα, lamin-A, and β-actin. An IκBα fragment is present in infected cells. (B) HeLa cells were infected with CVB3 for 0–2 h or treated with TNF-α for 30 min, and nuclear extracts were immunoprecipitated with antibody to p65 and immunoblotted with antibody to p65 (Upper) or antibody to the N terminus of IκBα (Lower). CVB3 infection leads to the appearance of an NF-κB:IκBα complex in the nucleus. (C) Schematic of the primary structure of IκBα with potential consensus 3Cpro cleavage sites at amino acid residues 151–155 and 249–250. Q is the P1 residue and G is the P1′ residue of the consensus cleavage sites. SRD is the signal receiving domain. PEST is a domain rich in proline, glutamate, serine, and threonine residues. (D) 3Cpro cleaves IκBα in vitro. Recombinant GST- IκBα was incubated with recombinant (His)6-3Cpro, fractionated by SDS/PAGE, and immunoblotted with antibody to the N terminus of IκBα (lanes 2–4) or antibody to the C terminus of IκBα (lane 5). (E) 3Cpro cleavage of IκBα mutants in vitro. WT or mutant 35S-labeled recombinant IκBα was incubated with recombinant 3Cpro, fractionated by SDS/PAGE, and autoradiographed. 3Cpro cleaves IκBα(WT) and IκBα(Q154G) but not IκBα(Q249G).
The CVB3 Protease 3Cpro Cleaves IκBα.
We postulated that the IκBα-immunoreactive fragment detected after Coxsackievirus infection is generated by a viral protease cleaving IκBα. The primary structure of IκBα contains two consensus sequences for the CVB3 viral protease 3Cpro: AXXQG at residues 151–155 and QG at residues 249–250 (Fig. 3C). To determine whether or not 3Cpro can cleave IκBα, we incubated recombinant 3Cpro with the recombinant 65-kDa fusion polypeptide GST-IκBα and immunoblotted the mixture with antibodies to IκBα. The 3Cpro protease cleaves recombinant IκBα in vitro (Fig. 3D), as detected by immunoblotting with antibody to the amino terminus of IκBα (Fig. 3D, lanes 2–4), and with antibody to the carboxyl terminus of IκBα (Fig. 3D, lane 5). As a control, a partially inactive catalytic mutant 3Cpro(C147S) was unable to cleave IκBα with high efficiency (Fig. 8, which is published as supporting information on the PNAS web site) (11).
To determine the precise location at which 3Cpro cleaves IκBα, we constructed mutant IκBα polypeptides lacking either one of the two 3Cpro recognition sequences, IκBα(Q154G) or IκBα(Q249G). The 3Cpro protease cleaves WT IκBα and mutant IκBα(Q154G) (Fig. 3E). However, 3Cpro protease cannot cleave mutant IκBα(Q249G), suggesting that 3Cpro cleaves IκBα between Q249 and G250 (Fig. 3E).
The IκBα Fragment Enters the Nucleus and Retains p65 in the Nucleus.
We next defined the location of the IκBα fragment in the cell. We transfected HeLa cells with empty vector (control), vector expressing WT IκBα [IκBα(WT)], or vector expressing the amino-terminal fragment of IκBα generated by the viral protease [IκBα(1–249)]. We treated these transfected cells with TNF-α to trigger NF-κB translocation to the nucleus, and then washed away the TNF-α to allow the cells to return to rest. We then monitored the location of the NF-κB p65 subunit and IκBα.
Expression of the IκBα fragment changes the location of NF-κB. In control cells, NF-κB normally is found in the cytoplasm; NF-κB translocates to the nucleus after TNF-α treatment, and then NF-κB is found in the cytoplasm again after TNF-α is washed away (Fig. 4A Top). Expression of WT IκBα does not change this normal pattern NF-κB localization (Fig. 4A Middle). However, expression of the IκBα(1–249) fragment causes NF-κB to remain in the nucleus after TNF-α is washed away (Fig. 4A Bottom).
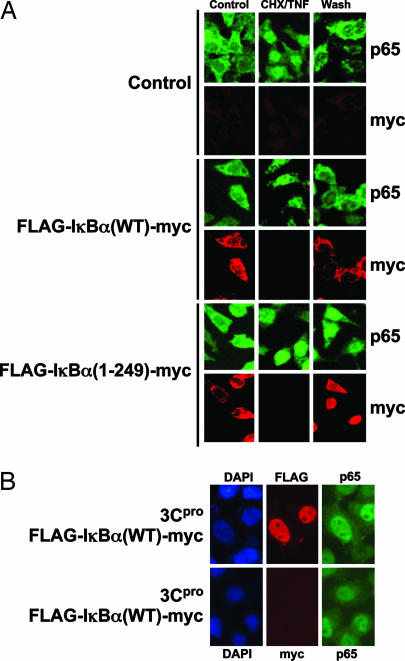
Fig. 4.
The IκBα fragment enters the nucleus and retains NF-κB in the nucleus. (A) HeLa cells were transfected or not with a plasmid expressing the fusion protein FLAG-IκBα(WT)-myc or an IκBα fragment consisting of FLAG-IκBα-(1–249)-myc, which corresponds to the IκBα fragment generated by the viral protease. Transfected cells were washed with PBS, preincubated 90 min in serum-free medium with 100 μg/ml cyclohexamide (CHX), and then treated for 30 min with 10 ng/ml TNF-α plus 100 μg/ml CHX (CHX/TNF). The cells were then washed and subsequently incubated for 90 min in the absence of serum (Wash). Cells were fixed, permeabilized, and assayed for p65 (FITC, green) and IκBα (Cy3, red) by confocal microscopy (n = 3). (B) HeLa cells were transfected with a plasmid expressing 3Cpro and cotransfected with a plasmid expressing the fusion protein FLAG- IκBα(WT)-myc. Cells were treated as before and assayed for the FLAG-tagged amino-terminal IκBα fragment or the myc-tagged carboxyl-terminal IκBα fragment (red) and for p65 (green) (n = 3). (Magnification: ×60.)
Furthermore, the location of the IκBα fragment is different from the location of the full-length IκBα. The WT IκBα is found in the cytoplasm of resting cells (Fig. 4A Middle). IκBα disappears from the cell during TNF-α treatment, and then IκBα accumulates again in the cytoplasm after TNF-α is removed (Fig. 4A Middle). In contrast, the IκBα fragment is found in the nucleus instead of the cytoplasm after TNF-α is washed away (Fig. 4A Bottom).
In summary, our data show that an IκBα fragment is localized to the nucleus, and that expression of this fragment causes NF-κB to remain in the nucleus.
The Viral Protease Generates an IκBα Fragment That Remains in the Nucleus.
To prove that the viral protease generates an IκBα fragment, we overexpressed the viral protease 3Cpro in HeLa cells. We also expressed the full-length IκBα and tagged the IκBα at the amino terminus with FLAG and at the carboxyl terminus with c-myc. We again stimulated the transfected cells with TNF-α and followed the location of the amino terminus and carboxyl terminus of IκBα.
The viral protease 3Cpro leads to the appearance of the amino-terminus FLAG tag but not the carboxyl-terminus myc tag (Fig. 4B). This finding suggests that the viral protease generates an amino-terminal fragment of IκBα. Furthermore, the FLAG epitope is found in the nucleus, supporting the idea that the IκBα fragment translocates into the nucleus (Fig. 4B). Finally, the viral protease also causes NF-κB to colocalize with IκBα in the nucleus (Fig. 4B). In contrast, expression of full-length IκBα without the viral protease leads to normal NF-κB localization in the cytoplasm (Fig. 4A Middle). A partially inactive mutant 3Cpro(C147S) did not induce NF-κB translocation to the nucleus (Fig. 9, which is published as supporting information on the PNAS web site) (11).
Thus the viral protease generates an amino-terminal IκBα fragment that colocalizes with NF-κB in the nucleus.
The IκBα Fragment Increases Apoptosis.
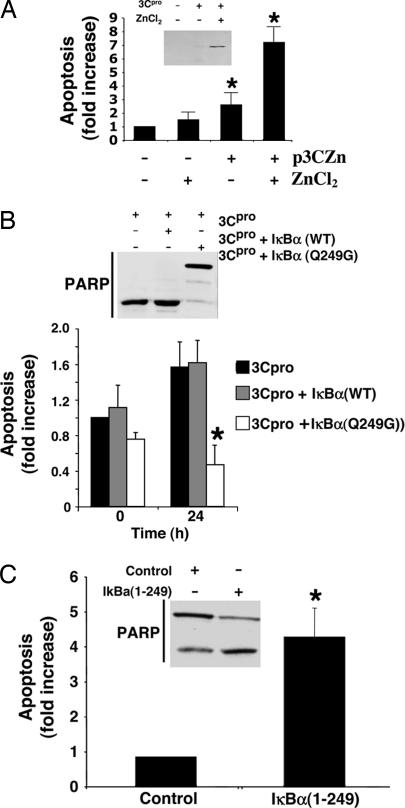
We next explored the functional consequences of viral protease cleavage of IκBα. We hypothesized that viral infection causes apoptosis by the viral protease cleaving IκBα. To test this idea, we first constructed a mammalian expression vector pZn3Cpro that encodes the Coxsackievirus protease 3Cpro under control of a zinc-responsive promoter (Fig. 5A). Expression of 3Cpro causes apoptosis of HeLa cells (Fig. 5A). We then expressed 3Cpro in HeLa cells transfected with a vector expressing IκBα(WT) or cleavage-resistant IκBα(Q249G), and then measured apoptosis.
Fig. 5.
Viral protease generates an IκBα fragment that causes apoptosis. (A) 3Cpro induces apoptosis in HeLa cells. HeLa cells were transiently transfected with the pZn3Cpro vector, which encodes 3Cpro regulated by a Zn-responsive promoter element. ZnCl2 treatment induces 3Cpro expression (Inset). HeLa cells transfected with pZn3Cpro and treated with ZnCl2 were assayed for apoptosis. Expression of the 3Cpro protease causes apoptosis (n = 3 ± SD; ∗, P < 0.05). (B) Cleavage-resistant IκBα(Q249G) inhibits protease-induced apoptosis. HeLa cell lines expressing WT IκBα or mutant IκBα(Q249G) were transiently transfected with pZn3Cpro, treated with ZnCl2 for 0 or 24 h, and assayed for apoptosis by FACS [n = 3 ± SD; ∗, P < 0.05 vs. IκBα(WT)], and by PARP cleavage (Inset). (C) The IκBα fragment activates apoptosis. HeLa cells were transfected with an empty control vector or a vector expressing the IκBα fragment. Transfected cells were stimulated with TNF-α for 30 min and then apoptosis was measured by FACS and PARP cleavage (Inset). n = 3 ± SD; ∗, P < 0.05.
The viral protease expression activates apoptosis, but expression of cleavage-resistant IκBα blocks apoptosis (Fig. 5B). The ability of the viral protease to cause apoptosis is limited by expression of a mutant IκBα that is resistant to protease cleavage. This finding suggests that the viral protease activates apoptosis in part by cleaving IκBα. These data also suggest that the IκBα fragment cleaved by the viral protease increases apoptosis of infected cells. To demonstrate that the IκBα fragment can cause apoptosis, we transiently expressed IκBα(WT) (control) or the amino-terminal fragment of IκBα [IκBα(1–249)] in HeLa cells and measured apoptosis. The IκBα fragment increases apoptosis (Fig. 5C). This result suggests that 3Cpro induces apoptosis in part through proteolysis of IκBα.
IκBα Increases Apoptosis During Viral Infection.
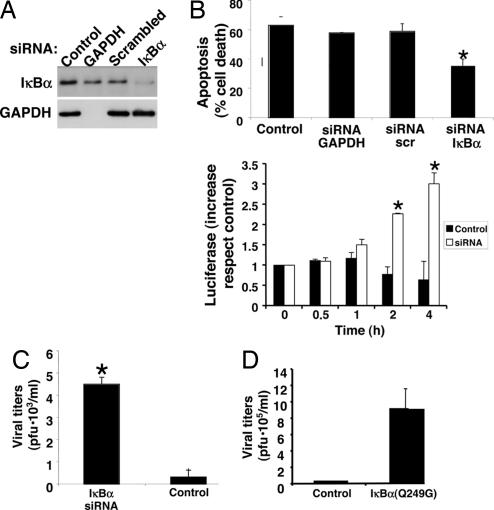
To support the hypothesis that IκBα plays a critical role in the host response to Coxsackievirus infection, we silenced IκBα expression by RNA interference (Fig. 6A). Silencing IκBα expression decreases apoptosis induced by viral infection (Fig. 6B Upper) and increases NF-κB activity (Fig. 6B Lower). Silencing IκBα expression also permits an increase in viral replication (Fig. 6C). Finally, expressing a cleavage-resistant IκBα permits an increase in viral replication (Fig. 6D). These data suggest that IκBα regulates apoptosis during viral infection.
Fig. 6.
IκBα regulates apoptosis in infected cells. (A) RNA silencing of IκBα. HeLa cells were transfected or not with dsRNA oligonucleotides specific for GAPDH, control scrambled oligonucleotides, or oligonucleotides for IκBα. The expression of IκBα (Upper) and GAPDH (Lower) were detected by immunoblotting. (B) (Upper) RNA silencing of IκBα decreases apoptosis in infected cells. HeLa cells were transfected with siRNA oligonucleotides and infected with CVB3 for 10 h, and apoptosis was measured. (Lower) RNA silencing of IκBα increases NF-κB activity. Cells were transfected or not with siRNA for IκBα silencing, and then transfected with a κB-luciferase reporter construct. NF-κB activity was measured by luminometry at different time points. n = 3, ± SD; ∗, P < 0.05 vs. control. (C) RNA silencing of IκBα increases viral replication. CVB3 replication was measured after control or IκBα silenced cells were infected with CVB3 (multiplicity of infection = 1) for 10 h (n = 3 ± SD; ∗, P < 0.05). (D) Overexpression of a mutant IκBα resistant to cleavage permits an increase in viral replication. HeLa cells were transfected with a control vector or a vector expressing a cleavage-resistant IκBα and infected with CVB3, and viral titers were measured after 10 h (n = 3 ± SD).
Discussion
The major finding of our study is that a viral protease cleaves IκBα, generating an IκBα fragment. This IκBα fragment enters the nucleus, interacts with NF-κB, and inhibits NF-κB transactivation. As a consequence, the antiapoptotic effects of NF-κB are suppressed and apoptosis increases, thereby limiting viral replication. IκBα may thus act as a host sensor for viral infection.
Coxsackievirus and other Picornaviruses cause apoptosis of infected cells in vivo and in vitro (12–17). Although expression of individual enterovirus polypeptides can cause apoptosis in vitro, it is unclear precisely how these viral polypeptides cause apoptosis (18–21). Our study shows that the Coxsackievirus protease 3Cpro is sufficient to cause apoptosis, in part by cleaving IκBα.
Several lines of evidence suggest that the viral protease 3Cpro generates an IκBα fragment that interrupts NF-κB signaling. The viral protease generates an IκBα fragment both in vitro (Fig. 3D) and in cells (Fig. 3A and Fig. 4B). The IκBα fragment interacts with and retains NF-κB in the nucleus (Fig. 3B and Fig. 4). The IκBα fragment also increases apoptosis (Fig. 5C). Taken together, these data suggest that the IκBα fragment generated by the viral protease can interfere with NF-κB signaling.
Other laboratories have shown that host proteases can cleave IκBα. Activation of apoptosis by TNF-α leads to caspase-3 cleavage of IκBα in vitro, suppressing NF-κB signaling and sensitizing cells to apoptosis (22, 23). Our data extend these previous studies by showing that viral protease cleavage of IκBα can disrupt NF-κB signaling, facilitate apoptosis, and decrease viral replication.
Because NF-κB modulates many innate and adaptive immune pathways, disruption of NF-κB signaling would be expected to have multiple consequences to the infected host. NF-κB signaling may play a dual role during viral infection of the host. In noninfected cells activated by adjacent infected cells, activation of NF-κB pathways may induce transcription of sets of genes, leading to enhanced expression of immune effector molecules. It is also possible that in infected cells viral infection shuts down NF-κB-induced transcription of antiviral genes. Furthermore, in infected cells inactivation of NF-κB may decrease transcription of antiapoptotic genes, increasing apoptosis of infected cells and limiting viral replication. Our data fit with studies by Knowlton and coworkers (24) that show that survival of p50 null mice is greater than survival of WT mice after viral infection, in part because of increased apoptosis and decreased viral replication. These data of Knowlton and coworkers emphasize the importance of NF-κB in regulating apoptosis during viral infection in vivo. The current study suggests that inactivation of NF-κB signaling in infected cells is a direct consequence of viral protease processing of IκBα.
The innate immune system recognizes pathogen-associated molecular patterns (PAMPs) that are necessary for the survival of specific pathogens. Viral proteases are PAMPs that play a critical role in the life cycle of Picornaviruses and many other RNA viruses. Susceptibility to viral proteases may be a novel mechanism by which the host recognizes and responds to viral infections.
Methods
Cells and Viruses.
CVB3 (Nancy strain) was propagated in HeLa cells as described (13). The cells were cultured in MEM (Invitrogen, Carlsbad, CA) supplemented with 1% l-glutamate (100 mM), 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. Infected cells were fed in medium with 1% serum. Viral stocks were prepared by the infection of 150-cm2 flask, 80–90% confluent monolayer culture of HeLa cells, at a multiplicity of infection of 1. Two days after incubation at 37°C, the cells were frozen and thawed three times, and the suspension was centrifuged. Viral supernatants were titered and stored at −80°C. Virus titers were performed by the plaque assay method (13). CVB3 stock titer was of 2 × 109 pfu/ml.
Assays for Apoptosis.
Apoptosis was measured by several complementary techniques, including DAPI staining (Molecular Probes, Carlsbad, CA), immunoblotting for PARP (a caspase substrate) detection by immunoblot, an ELISA that detects oligonucleotides and histones released as result of apoptosis (Cell Death Detection ELISA; Roche, Indianapolis, IN), and flow cytometry analysis (FACS) using propidium iodide (Sigma, St. Louis, MO) and annexin V-FITC (Dakocytomation, Fort Collins, CO), following the manufacturer's instructions.
Western Blot Analysis.
Cells were lysed in 1× lysis buffer (50 mM Tris, 1 mM EDTA, 1 mM EGTA, 0.17 μg/ml PMSF, and 2 μg/ml protein inhibitors: leupeptin, pepstatin, antipain, and antitrypsin). The homogenate was briefly centrifuged, and supernatants were collected. Proteins were quantified by Coomassie assay, and the samples were stored at −20°C. Fifty micrograms of cell homogenate was fractionated by SDS/PAGE and then transferred to a nylon membrane, blocked, incubated with primary antibodies and with secondary antibodies conjugated to HRP, and detected by chemiluminescence (ECL; Amersham, Piscataway, NJ). Human anti-PARP monoclonal antibody was from Trevigen (Gaithersburg, MD). Polyclonal anti-p65, IκBα antibodies, polyclonal anti-Bcl2, polyclonal anti-Bcl-XL, polyclonal anti-c-myc, polyclonal anti-lamin-A, polyclonal anti-β-actin, and monoclonal anti-GST antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-FLAG was from Sigma.
Immunoprecipitation.
Extracts from whole lysates or nuclear purified were precleared with control IgG corresponding to the host of the primary antibody, together with protein A-Sepharose. After centrifugation, the cell lysate was incubated overnight with the appropriate antibody and protein A-Sepharose. The pellets were collected by centrifugation, washed three times with RIPA buffer (0.15 mM NaCl/0.05 mM Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS), and resuspended with electrophoresis sample buffer.
Immunocytochemistry.
HeLa cells were grown in cover slides and incubated with anti-p65 (Santa Cruz Biotechnology) and anti-N-terminal-IκBα (Santa Cruz Biotechnology) or anti-p65 and anti-C-terminal-IκBα (Santa Cruz Biotechnology) antibodies for 2 h. Covers were washed twice with PBS-0.1% BSA and then incubated with anti-FITC (p65, green), anti-Cy3 (IkB, red), and Hoecht (nuclear detection, blue) for 1 h. Covers were washed four times with PBS-0.1% BSA and mounted in microscope slides for confocal microscopy visualization of the proteins.
Expression and Purification of Recombinant Proteins.
CVB3 3Cpro was expressed in bacteria and purified as described (11). In brief, we constructed a prokaryotic expression vector encoding a fusion polypeptide encoding (His)6-3Cpro (11). CVB3(His)6-3Cpro was expressed in bacteria and purified by metal chelation chromatography, dialyzed, and stored at −80°C.
The IκBα cDNA was kindly provided by Sankar Ghosh (Yale University, New Haven, CT). Mutant IκBα expression vectors were constructed by site-directed mutagenesis using a kit (Stratagene, La Jolla, CA). Oligonucleotide primers used to generate mutant IκBα constructs were: IκBα(Q154G) primer, 5′-GCCTGTGAGGGGGGCT-3′; IκBα(Q249G) primer, 5′-GTTACCTACGGGGGC-3′. IκBα(WT), IκBα(Q154G), and IκBα(Q249G) were expressed and 35S-methionine-radiolabeled by in vitro transcription and translation using the TNT Quick coupled transcription/translation system (Promega, Madison, WI).
GST-truncated IκBα, which lacks the last 68 aa of WT IκBα, was generated by PCR, and the resulting fragment was cloned into pGEX4T2 (GE-Amersham, Piscataway, NJ). The fragment was expressed in bacteria and purified by affinity chromatography, dialyzed, and stored at −80°C.
Flag-IκBα-myc, and Flag-IκBαT-myc were generated by PCR and cloned in p3XFLAG-myc (Sigma) for eukaryotic expression.
Transient Transfection of Cells.
HeLa cells were transiently transfected with 1 μg of DNA and 10 μl of Lipofectamine 2000 for 4 h, washed, and incubated with MEM/10% FCS for 16 h. The zinc-inducible 3Cpro plasmid (pZn3Cpro) was constructed by ligating the coding sequence of CVB3 3Cpro into the multiple cloning site of the mammalian expression vector pMEP4 (Invitrogen). Expression of 3Cpro in HeLa cells transfected with this vector was induced with 100 μM ZnCl2. NF-κB transactivation was monitored by measuring luciferase activity expressed from a reporter vector containing the luciferase coding sequence driven by three κB elements. Cells were cotransfected with a κB promoter fused to a Photinus luciferase construct and also with a construct containing the constitutive promoter region of CMV fused to a R. luciferase gene. Every sample was then analyzed for P. luciferase content and also for R. luciferase, whose expression is independent of infection. Results were normalized by R. luciferase content.
Stable Transfection of HeLa Cells.
The cDNA encoding WT and mutant IκBα were subcloned into pCDNA3.1 (Invitrogen). To generate the stable cell lines that express IκBα, six-well plates of HeLa cells were incubated with 2 μg of plasmid DNA mixed with the Lipofectamine 2000 reagent (Invitrogen) for 48 h and then selected in media containing G418. After selection, the cells were maintained with 0.5 mg/ml of G418.
In Vitro Digestion of IκBα by 3Cpro.
Recombinant GST-IκBα (Santa Cruz Biotechnology) or in vitro-transcribed and -translated IκBα was incubated with affinity-purified 3Cpro indigestion buffer (50 mM Tris·HCl, pH 7.5/0.1 M NaCl/1 mM EDTA) for 16 h at 30°C, fractionated by SDS/PAGE, and then analyzed by immunoblotting for IκBα or autoradiographed.
RNA Interference.
We performed gene silencing of IκBα and GAPDH by transfecting HeLa cells with human IκBα and GAPDH-validated siRNAs (Ambion, Austin, TX). Transfection was performed with Lipofectamine 2000 according to the manufacturer's instructions. Gene silencing was monitored by immunoblot of cell extracts isolated 24, 48, and 72 h after transfection. Controls were performed by transfection with a GAPDH siRNA or a scrambled siRNA (Ambion).
Statistical Analysis.
All experiments were performed in triplicate, with the variability of data described by ± SD. Comparisons were made with analysis of variance followed by Dunnett′s modification of the t test, whenever comparisons were made with a common control. The level of statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL63706, HL074061, HL65608, HL078635, and HL056091 (to C.J.L.), American Heart Association Grant 0140210N (to C.J.L.), Ministry of Science and Technology of Spain Grants SAF2002-00399 and SAF2005-06025 (to C.Z.) and SAF2000-0149 (to S.L.), “Programa Ramon y Cajal” Ministerio de Ciencia Y Tecnologia (C.Z. and M.S.), the Ciccarone Center (C.J.L.), and the John and Cora H. Davis Foundation (C.J.L.).
Abbreviations
- IκBα
inhibitor of κBα
- PARP
poly(ADP-ribose) polymerase
- CVB3
Coxsackievirus B3.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Roulston A, Marcellus RC, Branton PE. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 2.Benedict CA, Norris PS, Ware CF. Nat Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZG, Hsu H, Goeddel DV, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Lin A. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Verma IM. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Karin M. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 7.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Beg AA, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Mayo MW, Baldwin AS., Jr Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 10.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 11.Saura M, Zaragoza C, McMillan A, Quick RA, Hohenadl C, Lowenstein JM, Lowenstein CJ. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henke A, Huber S, Stelzner A, Whitton JL. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaragoza C, Ocampo CJ, Saura M, McMillan A, Lowenstein CJ. J Clin Invest. 1997;100:1760–1767. doi: 10.1172/JCI119702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alter P, Jobmann M, Meyer E, Pankuweit S, Maisch B. Cardiovasc Pathol. 2001;10:229–234. doi: 10.1016/s1054-8807(01)00077-1. [DOI] [PubMed] [Google Scholar]
- 15.Seko Y, Kayagaki N, Seino K, Yagita H, Okumura K, Nagai R. J Am Coll Cardiol. 2002;39:1399–1403. doi: 10.1016/s0735-1097(02)01776-x. [DOI] [PubMed] [Google Scholar]
- 16.Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, Hunt DW, McManus BM. J Virol. 1998;72:7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girard S, Couderc T, Destombes J, Thiesson D, Delpeyroux F, Blondel B. J Virol. 1999;73:6066–6072. doi: 10.1128/jvi.73.7.6066-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstaub D, Gradi A, Bercovitch Z, Grosmann Z, Nophar Y, Luria S, Sonenberg N, Kahana C. Mol Cell Biol. 2000;20:1271–1277. doi: 10.1128/mcb.20.4.1271-1277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barco A, Feduchi E, Carrasco L. Virology. 2000;266:352–360. doi: 10.1006/viro.1999.0043. [DOI] [PubMed] [Google Scholar]
- 20.Li ML, Hsu TA, Chen TC, Chang SC, Lee JC, Chen CC, Stollar V, Shih SR. Virology. 2002;293:386–395. doi: 10.1006/viro.2001.1310. [DOI] [PubMed] [Google Scholar]
- 21.Kuo RL, Kung SH, Hsu YY, Liu WT. J Gen Virol. 2002;83:1367–1376. doi: 10.1099/0022-1317-83-6-1367. [DOI] [PubMed] [Google Scholar]
- 22.Barkett M, Xue D, Horvitz HR, Gilmore TD. J Biol Chem. 1997;272:29419–29422. doi: 10.1074/jbc.272.47.29419. [DOI] [PubMed] [Google Scholar]
- 23.Reuther JY, Baldwin AS., Jr J Biol Chem. 1999;274:20664–20670. doi: 10.1074/jbc.274.29.20664. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz EM, Badorff C, Hiura TS, Wessely R, Badorff A, Verma IM, Knowlton KU. J Virol. 1998;72:5654–5660. doi: 10.1128/jvi.72.7.5654-5660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.