Abstract
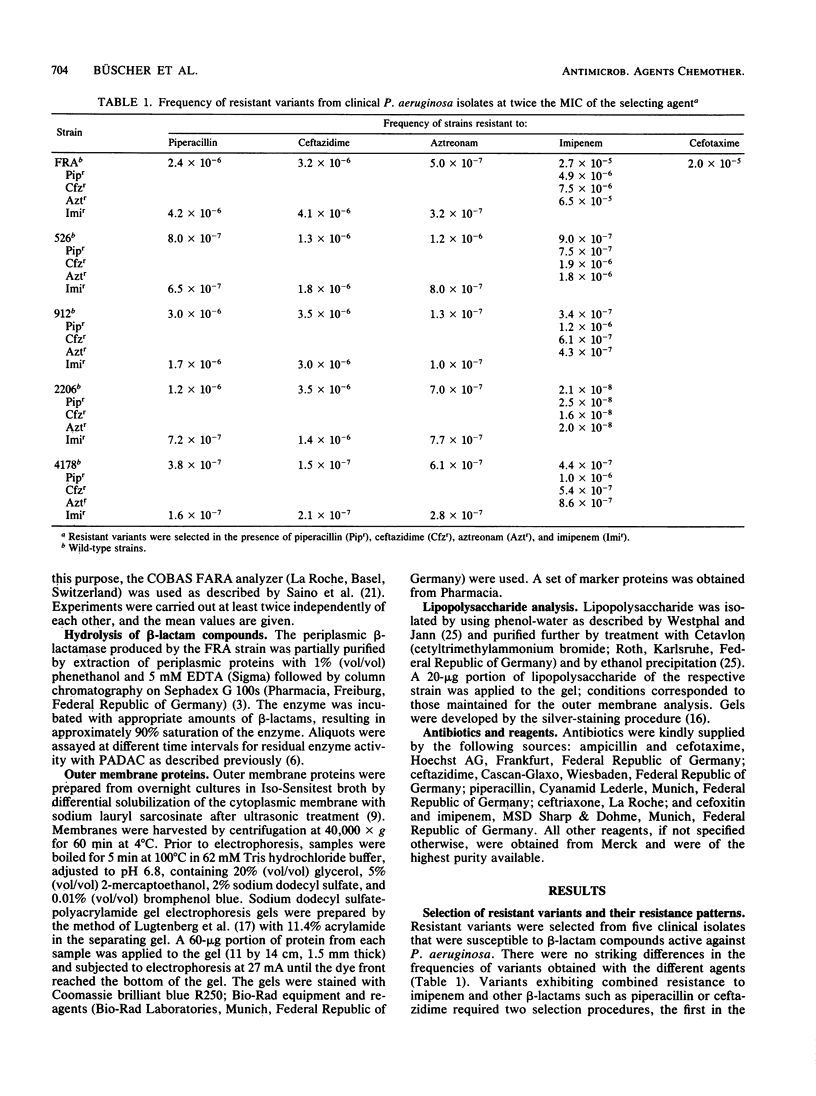
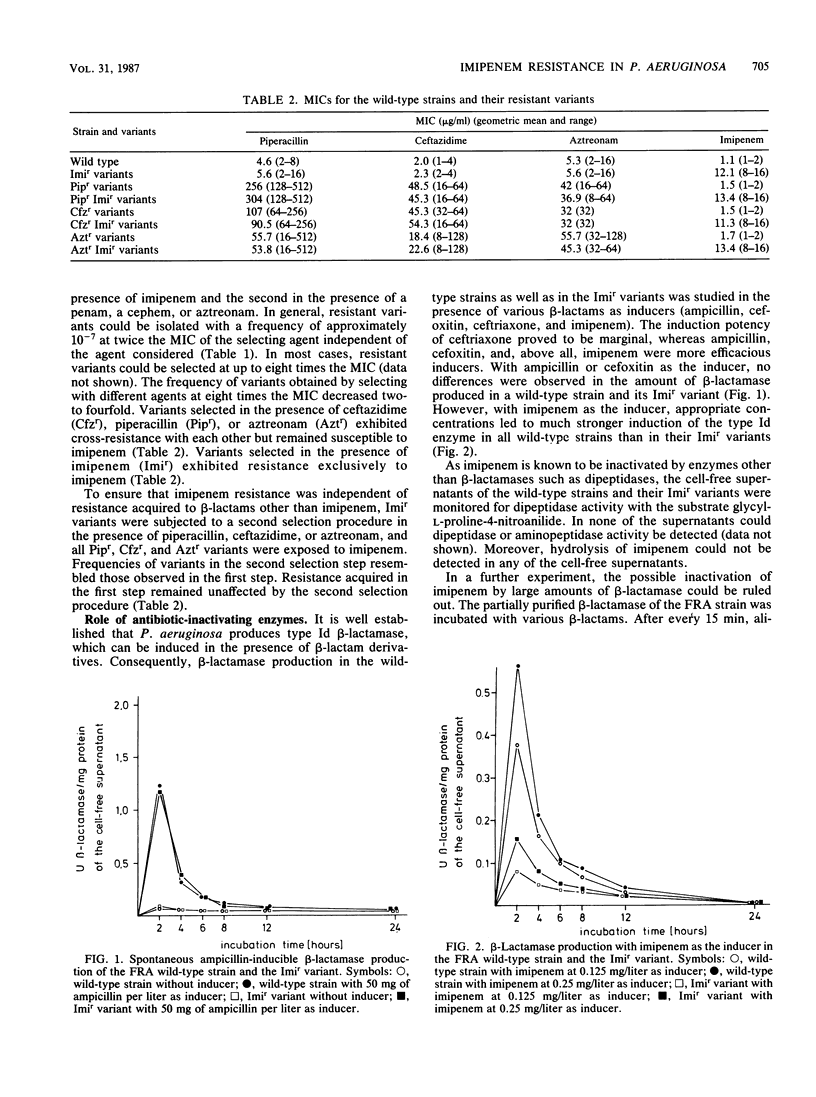
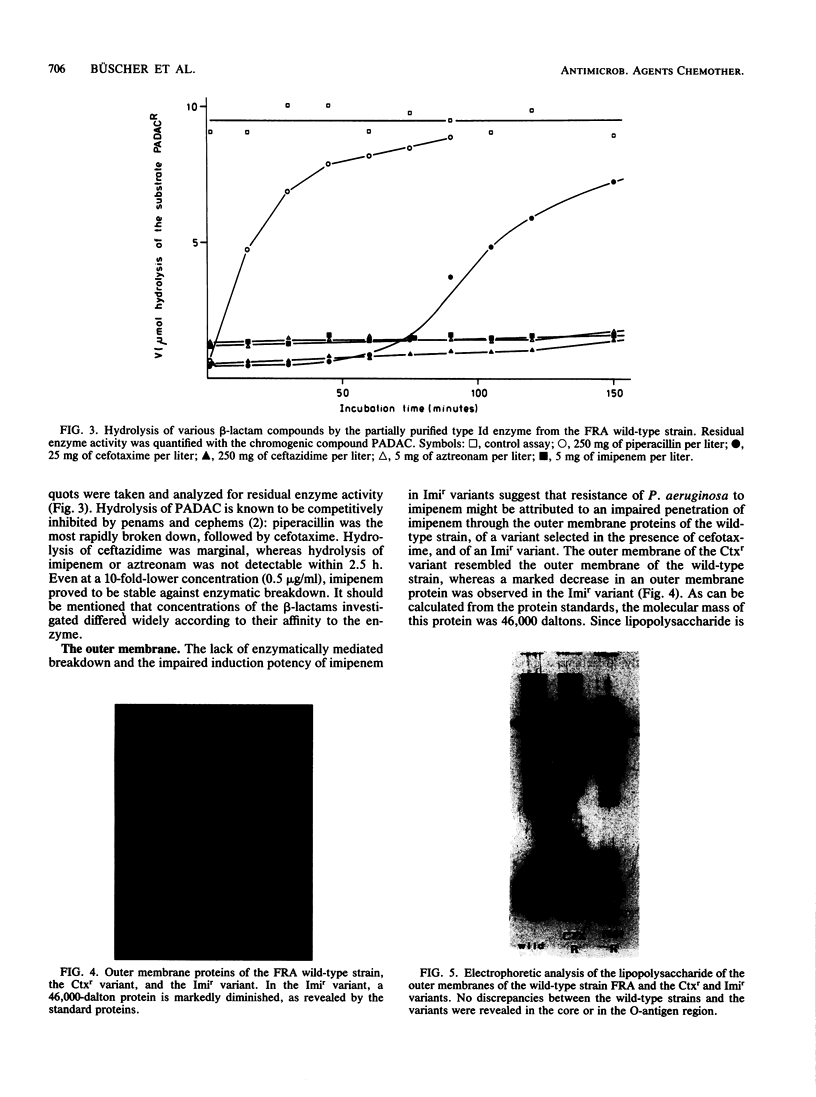
The mechanism of Pseudomonas aeruginosa resistance to imipenem in five imipenem-susceptible clinical isolates and in their resistant counterparts was investigated. The frequency for selecting imipenem-resistant variants ranged from 2.7 X 10(-5) to 2.1 X 10(-8) and was comparable to those for other beta-lactams. Cross-resistance between imipenem and other beta-lactam compounds was not observed. In all imipenem-resistant variants, induction of chromosomal beta-lactamase by imipenem was markedly diminished compared with that in the susceptible parent strain. This was not the case for other inducers such as ampicillin or cefoxitin, suggesting an impaired uptake of imipenem as an explanation for resistance. Analysis of the outer membrane proteins revealed a marked decrease of either a 46- or a 45-kilodalton protein. The lipopolysaccharide of the outer membrane in the imipenem-resistant variants was not altered.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F. Therapy for lower respiratory tract infections with imipenem/cilastatin: a review of worldwide experience. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S513–S517. doi: 10.1093/clinids/7.supplement_3.s513. [DOI] [PubMed] [Google Scholar]
- Berks M., Redhead K., Abraham E. P. Isolation and properties of an inducible and a constitutive beta-lactamase from Pseudomonas aeruginosa. J Gen Microbiol. 1982 Jan;128(1):155–159. doi: 10.1099/00221287-128-1-155. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Dalhoff A., Dick W. Nonspecific induction of beta-lactamase in Enterobacter cloacae. J Gen Microbiol. 1984 Jul;130(7):1781–1786. doi: 10.1099/00221287-130-7-1781. [DOI] [PubMed] [Google Scholar]
- Cullmann W., Dick W. Investigations on beta-lactamase stability of recently developed beta-lactam compounds: study of enzyme kinetics. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):413–422. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer E. H., Donabedian H., Raeder R., Ribner B. S. Empirical use of imipenem as the sole antibiotic in the treatment of serious infections. J Antimicrob Chemother. 1985 Oct;16(4):499–507. doi: 10.1093/jac/16.4.499. [DOI] [PubMed] [Google Scholar]
- Fujita K., Hirano M., Tokunaga K., Nagatsu I., Nagatsu T., Sakakibara S. Serum glycylproline p-nitroanilidase activity in blood cancers. Clin Chim Acta. 1977 Dec 1;81(2):215–217. doi: 10.1016/0009-8981(77)90014-6. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Pedersen S. S., Pressler T., Høiby N., Bentzon M. W., Koch C. Imipenem/cilastatin treatment of multiresistant Pseudomonas aeruginosa lung infection in cystic fibrosis. J Antimicrob Chemother. 1985 Nov;16(5):629–635. doi: 10.1093/jac/16.5.629. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbore D., Pontzer R., Levison M. E., Kaye D., Cynamon M., Liu C., Hinthorn D. R., Tan J. S., File T. M. Multicenter study of the clinical efficacy of imipenem/cilastatin for treatment of serious infections. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S476–S481. doi: 10.1093/clinids/7.supplement_3.s476. [DOI] [PubMed] [Google Scholar]