Abstract
Regional alveolar hypoxia causes local vasoconstriction in the lung, shifting blood flow from hypoxic to normoxic areas, thereby maintaining gas exchange. This mechanism is known as hypoxic pulmonary vasoconstriction (HPV). Disturbances in HPV can cause life-threatening hypoxemia whereas chronic hypoxia triggers lung vascular remodeling and pulmonary hypertension. The signaling cascade of this vitally important mechanism is still unresolved. Using transient receptor potential channel 6 (TRPC6)-deficient mice, we show that this channel is a key regulator of acute HPV as this regulatory mechanism was absent in TRPC6−/− mice whereas the pulmonary vasoconstrictor response to the thromboxane mimetic U46619 was unchanged. Accordingly, induction of regional hypoventilation resulted in severe arterial hypoxemia in TRPC6−/− but not in WT mice. This effect was mirrored by a lack of hypoxia-induced cation influx and currents in smooth-muscle cells from precapillary pulmonary arteries (PASMC) of TRPC6−/− mice. In both WT and TRPC6−/− PASMC hypoxia caused diacylglycerol (DAG) accumulation. DAG seems to exert its action via TRPC6, as DAG kinase inhibition provoked a cation influx only in WT but not in TRPC6−/− PASMC. Notably, chronic hypoxia-induced pulmonary hypertension was independent of TRPC6 activity. We conclude that TRPC6 plays a unique and indispensable role in acute hypoxic pulmonary vasoconstriction. Manipulation of TRPC6 function may thus offer a therapeutic strategy for the control of pulmonary hemodynamics and gas exchange.
Keywords: hypoxia-induced diacylglycerol accumulation, precapillary pulmonary arterial smooth-muscle cells, pulmonary hypertension, transient receptor potential channel 6-deficient mouse model, arterial hypoxemia
Acute regional hypoxic pulmonary vasoconstriction (HPV) is necessary to maintain optimized gas exchange by directing blood flow from poorly ventilated to well ventilated areas of the lung. Under conditions of generalized hypoxia, however, total pulmonary vascular resistance rises with subsequent increase of right heart load (1–3). Chronic hypoxia, as occurring in ventilatory disorders induces chronic pulmonary hypertension, pulmonary vascular remodeling, and cor pulmonale (4). The underlying oxygen sensing and signal transduction mechanisms of the acute and chronic vascular responses are largely unknown. A rise of intracellular calcium ([Ca2+]i) in pulmonary artery smooth-muscle cells (SMCs) has been suggested to be the key event in these processes (5–8). However, the question how [Ca2+]i is regulated has not yet been resolved. Among others, transient receptor potential (TRP) channels are regulators of [Ca2+]i. The TRP protein superfamily consists of a diverse group of nonselective cation channels involved in many basic cellular processes (9). Whereas members of the TRPV and TRPM subfamilies have emerged as versatile cellular sensors, the functional importance of the seven members (TRPC1 to -7) of the TRPC (transient receptor potential cation channel subfamily C) subfamily is still poorly understood. Among the TRPC channels, TRPC3, -6, and -7 are 75% identical and gated by pathways that activate C-type phospholipases (PLCs) as well as by direct exposure to diacylglycerols (DAGs) (10). Although TRPC6 is expressed in many smooth-muscle tissues (11) and is thought to be an essential regulator of smooth-muscle contractility (12–14), direct evidence for a unique, nonredundant physiological role in the pulmonary circulation is still lacking. However, recently an up-regulation of TRPC6 has been shown in lung tissue from patients suffering from chronic pulmonary hypertension (15).
In the present study, we investigated the function of TRPC6 channel activity in acute, prolonged, and chronic responses to alveolar hypoxia in intact WT and TRPC6-deficient animals, isolated lungs, and primary cultured precapillary pulmonary arterial smooth-muscle cells (PASMCs). We demonstrate that TRPC6 is essential for acute hypoxic pulmonary vasoconstriction but is not involved in prolonged or chronic lung vascular responses to hypoxia. Thus, TRPC6 may present a target for a molecular intervention to control pulmonary hemodynamics and gas exchange.
Results and Discussion
Acute and Sustained Hypoxic Pulmonary Vasoconstriction in TRPC6−/− Mice.
We analyzed the hypoxia-induced pressor response in isolated lungs from WT and TRPC6-deficient mice (TRPC6−/−). Ventilation of lungs from WT mice with 1% O2 provoked a biphasic vasoconstrictor response with a first maximum occurring within 10 min, followed by a pressure nadir and a second vasoconstrictor response developing after 60 min of hypoxic ventilation (Fig. 1A). The first (acute) phase of HPV was completely absent in TRPC6−/− mice, whereas the second (prolonged) vasoconstrictor response was not significantly different from that in WT mice (Fig. 1A). The lack of acute HPV in TRPC6−/− mice was not caused by a general defect in muscular contractility, as vasoconstriction induced by the thromboxane mimetic U46619 was not suppressed (Fig. 1B). Normoxic vascular tone did not differ between WT and TRPC6−/− mice [supporting information (SI) Fig. 5A. The lack of acute HPV in TRPC6−/− mice has profound physiological relevance, because partial occlusion of alveolar ventilation in anesthetized mice provoked severe hypoxemia in TRPC6−/− but not in WT mice (Fig. 1C).
Fig. 1.
Acute and prolonged HPV and arterial oxygenation in WT and TRPC6-deficient (TRPC6−/−) mice. (A) Time course of hypoxic vasoconstriction in isolated, buffer-perfused, and ventilated mouse lungs during 160 min of hypoxic ventilation. Changes in pulmonary artery pressure (ΔPAP) are shown for lungs from WT (filled circles, n = 5) and TRPC6−/− (open circles, n = 6) mice ventilated with a hypoxic gas of 1% O2. Control lungs were ventilated normoxically [WT, black triangles, n = 6; TRPC6−/−, white triangles, n = 5; ∗1, significant difference (P < 0.05) between WT and TRPC6−/− mice after applying acute hypoxia; ∗2, significant differences (P < 0.05) between normoxic (WT and TRPC6−/−) and hypoxic (WT and TRPC6−/−) mice]. (B) Specificity of TRPC6 for the hypoxia-induced vasoconstrictor response. Lungs of WT (filled bars) and TRPC6−/− (open bars) mice were challenged either with hypoxic ventilation (1% O2, 10 min, acute hypoxia) or with injection of the thromboxane mimetic U46619 into the pulmonary artery (U46619). ΔPAP, maximum increase in pulmonary artery pressure. Data are from n = 5 mice per group; ∗, significant difference (P < 0.05) between WT and TRPC6−/− mice. (C) Arterial oxygenation in anesthetized WT (filled circles) and TRPC6−/− (open circles) mice after provocation of regional ventilatory failure. Mice were ventilated with room air and challenged with an airway fluid load of 25 μl of saline by tracheal administration at time point zero. Arterial oxygenation was measured during the following 10 min. ∗, significant difference (P < 0.05) between WT and TRPC6−/− mice. Data are from n = 4 mice per group.
Exposure of WT mice to chronic hypoxia (10% O2) for 3 weeks induced pulmonary hypertension as evident from an increased right ventricular systolic pressure assessed in the anesthetized mouse, an increased ratio of the right ventricular muscle to the left ventricular plus septum mass, and an increased muscularization of small pulmonary arterial vessels (SI Fig. 6). Compared with WT mice, no differences in the development of pulmonary hypertension were observed in TRPC6−/− mice exposed to chronic hypoxia (SI Fig. 6). The signaling mechanisms underlying the acute, prolonged and chronic effects of alveolar hypoxia on the pulmonary vasculature are still largely unknown (16–20). At present it is not clear whether the very acute hypoxic vasoconstrictor response (occurring within seconds), the prolonged response (occurring within hours), and the initiation of the structural vascular remodeling process in chronic hypoxia are regulated by identical or different mechanisms (3). Recently, TRPC6 was found to be up-regulated in hypoxia-induced, as well as idiopathic chronic pulmonary hypertension (8, 15). However, an indispensable role of TRPC6 in the development of hypoxia-induced chronic pulmonary hypertension can clearly be excluded by our study. Moreover, the acute hypoxic vasoconstrictor response is shown to be distinct from prolonged HPV, which is unaffected in TRPC6−/− mice. Thus, our findings allow for a clear distinction between the mechanisms underlying the acute and prolonged/chronic effects of hypoxia in the pulmonary circulation.
Isolation of PASMC and Expression of TRPC Subtypes in PASMC.
As TRPC6 deficiency might be compensated for by up-regulation of closely related members of the TRPC family (14), we analyzed the expression of TRPC subtypes in primary nonpassaged PASMCs isolated from pulmonary arteries of 30–150 μm as described in Materials and Methods. PASMC cultured for 5 days without passaging (SI Fig. 7) revealed expected SMC immunoreactivity with antibodies against α-smooth-muscle actin (SI Fig. 7A) and myosin heavy chain (SI Fig. 7B). The lack of immunoreactivity to primary antibodies against von Willebrand factor however, excludes the presence of endothelial cells (SI Fig. 7C). No immunoreactivity of PASMC was observed, if primary antibodies were omitted (SI Fig. 7D).
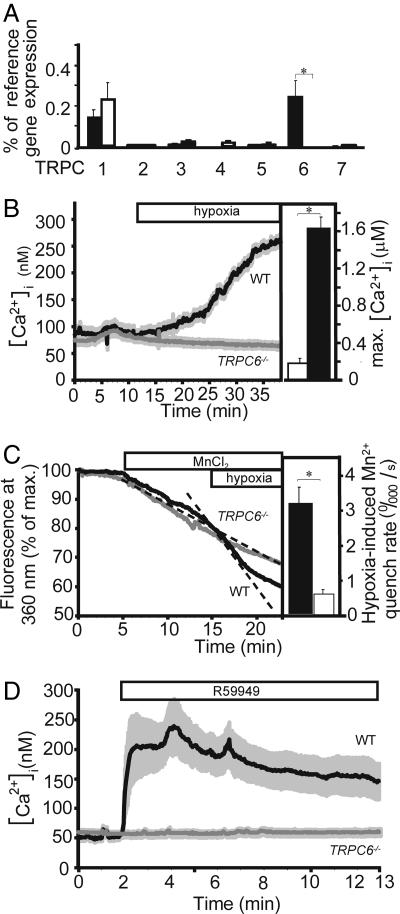
Quantitative RT-PCR-analysis revealed predominant expression of TRPC1 and TRPC6 mRNA in PASMC from WT mice. As expected, PASMC from TRPC6−/− mice did not express a complete TRPC6 mRNA. A compensatory up-regulation of other TRPC subtypes could be excluded (Fig. 2A).
Fig. 2.
TRPC expression analysis of, and cation influx in, PASMCs from WT and TRPC6−/− mice. (A) Total RNA was prepared from primary cultured precapillary pulmonary artery SMCs of WT (n = 3 mice) and TRPC6-deficient (TRPC6−/−, n = 3 mice) mice and reverse-transcribed. Products of the first strand synthesis were analyzed for the presence of amplification products obtained with primer pairs described in Materials and Methods. mRNAs coding for TRPCs and β-actin (as reference gene) were quantified with the aid of a light cycler. Values are presented as percentage of reference mRNA expression (β-actin mRNA expression). Except for TRPC6 expression (P < 0.05), no significant expression differences in WT and TRPC6−/− cells were observed (P > 0.05). (B–D) Cation influx in PASMCs from WT and TRPC6−/− mice. (B Left) Increase in [Ca2+]i upon exposure to hypoxia. Primary cultured PASMC were loaded with fura-2 and analyzed by single-cell fluorescence imaging. A horizontal bar indicates hypoxic superfusion of the cells. ET-1 (4 nM) was added 5 min after starting the experiment and was present during the remainder of the experiment. Data are from n = 15 WT and n = 55 TRPC6−/− cells from four mice each. (B Right) Maximal [Ca2+]i in PASMC (n = 6 cells) from TRPC6-deficient mice infected with AAVs coding for TRPC6-EGFP (a fusion protein of TRPC6 and the EGFP) before (open bar) and after (filled bar) application of hypoxia. ET-1 (4 nM) was added 5 min after starting the experiment and was present during the remainder of the experiment. (C Left) Hypoxia-induced Mn2+ influx in TRPC6−/− and WT PASMC. Mn2+ (0.3 mM) was added to the Ca2+-containing bath solution, and hypoxia was applied to PASMC from WT (n = 4 cells) and a TRPC6−/− (TRPC6−/−, n = 4 cells) mice as indicated by bars. ET-1 (4 nM) was added 5 min after starting the experiment and was present during the remainder of the experiment. Time courses of total fura-2 fluorescence at the isosbestic wavelength are shown. Total fura-2 fluorescence corrected for background signals was recorded by exciting fura-2 at the isosbestic wavelength (360 nm) and normalized to the initial values of each single cell. Dashed lines indicate the initial slopes, i.e., the rates of fura-2 quenching by Mn2+. (C Right) Summary of Mn2+ influx experiments. Differences in the linear range of the Mn2+ quench rate after Mn2+ addition and after application of hypoxic conditions were calculated for WT (filled bars, n = 12 cells) and TRPC6−/− PASMC (open bars, n = 22 cells). ∗ significant difference (P < 0.05) between WT and TRPC6−/− mice. (D) Effects of a DAG kinase inhibitor II (R59949) on [Ca2+]i in WT versus TRPC6−/− PASMC. ET-1 (4 nM) was added 1 min after starting the experiment and was present during the remainder of the experiment. The DAG kinase inhibitor R59949 induced a rise in [Ca2+]i in WT cells (n = 15) but not in TRPC6−/− cells (n = 75).
Hypoxia-Induced Cation Influx Depends on TRPC6 Expression in PASMC.
To examine the cellular mechanism of TRPC6-dependent HPV, we analyzed the hypoxia-induced increase in [Ca2+]i and membrane currents in PASMC. Hypoxic incubation of WT PASMC entailed an increase in [Ca2+]i after priming with endothelin-1 (ET-1, 4 nM) (Fig. 2B Left and SI Fig. 8 A and B) or angiotensin II (10 μM) (data not shown). This observation is in line with previous investigations of [Ca2+]i fluctuations in rat PASMC, where a priming with angiotensin II (21) was needed and the fact that, in isolated lungs from rats as well as in isolated pulmonary arterial rings or PASMC from different species, a “priming” with angiotensin II, ET-1, or PGF2a is a prerequisite to elicit any or full hypoxic vasoconstriction (22–24). The underlying mechanism of this priming is still not resolved (for review, see ref. 25).
The hypoxia-induced increase in [Ca2+]i was specific for PAMSC, because it was undetectable in ET-1-primed aortic SMCs (SI Fig. 8C). Most notably, the increase in [Ca2+]i elicited by hypoxic incubation of WT PASMC was absent in PASMC from TRPC6−/− mice (Fig. 2B Left). In contrast to TRPC6−/− aortic SMCs, where up-regulated TRPC3 increased cation influx (14), baseline [Ca2+]i values of isolated PASMC from WT and TRPC6−/− mice analyzed in calcium imaging experiments were not significantly different (SI Fig. 5B). The indispensable role of TRPC6 for acute HPV was further underlined by the recovery of the hypoxia-induced rise of [Ca2+]i subsequent to TRPC6 expression in PASMC from TRPC6−/− mice after infection with adeno-associated viruses (AAVs) coding for TRPC6-GFP (Fig. 2B Right). Notably, the Ca2+ increase in the PASMC did not show the biphasic kinetics of HPV in the isolated perfused lung (Fig. 1A), implicating that additional mechanisms in conjunction with intracellular Ca2+ levels underlie the biphasic response of HPV. Such mechanisms may include alterations in the Ca2+ sensitivity of the contractile apparatus and alterations in the level of vasodilators and constrictors as previously suggested (e.g., refs. 25 and 26).
As store-operated Ca2+ channels and capacitative calcium entry have been suggested to contribute to the regulation of HPV (5, 6, 27), we tested the role of internal Ca2+ stores in the hypoxia-induced increase in [Ca2+]i. The hypoxia-induced rise of [Ca2+]i was not dependent on a release from internal Ca2+ stores as hypoxia-induced increases in [Ca2+]i were not detectable in both WT and TRPC6−/− cells in the absence of extracellular Ca2+ (SI Fig. 9A), although both cells types reacted with a rise of [Ca2+]i in response to ET-1 (SI Fig. 9B). This finding contrasts with previous reports and may be explained by the fact that these investigators used pulmonary SMCs isolated from larger pulmonary arteries, which are not the physiological site of HPV (7, 8). However, Ca2+ release from internal stores may be relevant for the maintenance of [Ca2+]i levels during sustained HPV, probably involving cyclic ADP-ribose, as previously suggested (28).
Of note, the entry of Ca2+ ions in response to hypoxia is mostly carried by voltage-gated calcium channels, because nicardipine, a potent blocker of these channels, almost completely inhibited acute HPV in isolated lungs and Ca2+ influx in WT PASMC(SI Fig. 10). These data are fully consistent with a TRPC6 permeation model in which Ca2+ contributes only a small percentage (≈4%) to whole-cell currents in the presence of extracellular Na+ (29). The resulting Na+ influx through TRPC6 channels then leads to membrane depolarization and activation of voltage-gated calcium channels (29–31). Moreover, inhibition of potassium channels as a secondary event may also contribute to depolarization, because an elevation of intracellular Na+ is able to block potassium channels (32). To directly monitor TRPC6 activity in PASMC, we analyzed hypoxia-induced Mn2+ influx by fluorescence quenching in fura-2 loaded cells. In contrast to voltage-gated calcium channels, TRPC6 channels are permeable to Mn2+ ions. As expected, hypoxia initiated a cation influx from the extracellular space as shown by an increase in the Mn2+-quench rate after application of hypoxia in WT, but not in TRPC6−/− PASMC (Fig. 2C). To further elucidate signaling steps involved in TRPC6 channel activation, we prevented diacylglycerol degradation by addition of the DAG kinase inhibitor II (R59949) resulting in a rise of [Ca2+]i after priming with ET-1 in WT PASMC, whereas primed cells from TRPC6−/− mice were completely unresponsive (Fig. 2D; baseline Ca2+ levels are also given in SI Fig. 5B).
Hypoxia-Induced Membrane Currents Through TRPC6 in PASMC.
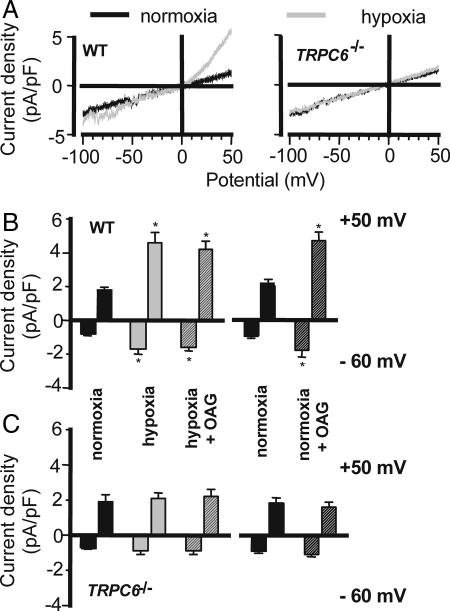
To directly assess membrane currents through TRPC channels resulting from hypoxia, we measured cationic currents in isolated PASMC using the whole-cell mode of the patch-clamp technique in the continuous presence of 10 μM nifedipine to block voltage-gated calcium channels. The cell membrane capacitance did not differ between PASMC derived from either source (14.6 ± 1.6 pF and 16.6 ± 0.8 pF for WT and TRPC6−/− cells, respectively). Ion currents were monitored in normoxic and hypoxic solutions by using voltage ramp commands in PASMC from WT and TRPC6−/− mice (Fig. 3A). Hypoxia increased both inward and outward currents in PASMC from WT mice, which is indicative of the hypoxia-induced activation of TRPC6-mediated cationic currents (Fig. 3 A Left and B). In contrast, the superfusion of cells with a hypoxic bath solution did not affect whole-cell currents recorded in PASMC from TRPC6−/− mice (Fig. 3 A Right and C). Under normoxic conditions, the exposure to OAG, a membrane-permeable analogue of DAG, resulted in a significant increase in current densities in PASMC from WT mice (Fig. 3B). In contrast, the effect of OAG on PASMC from TRPC6−/− mice was negligible, indicating that the OAG effect depends on TRPC6 (Fig. 3C). As OAG can also activate TRPC3, these findings are in line with our expression studies, excluding a significant contribution of TRPC3. Most interestingly OAG did not further enhance the hypoxia-induced increase in whole-cell currents in WT cells (Fig. 3B), suggesting that the effect of DAG/OAG and hypoxia converge upon the same signaling pathway.
Fig. 3.
Hypoxia-induced activation of TRPC6-mediated cationic current. (A) Representative traces of whole-cell currents in normoxic and hypoxic PASMC from WT (Left) and TRPC6-deficient (TRPC6−/−, Right) mice. (B and C) Summarized data of normalized currents elicited at a potential of −60 mV and +50 mV in normoxia (black bars) and after perfusion with hypoxic bath solution (gray bars) as well as after addition of the membrane-permeable analogue of diacylglycerol (OAG), during hypoxia (gray hatched bars; n = 7 for WT and n = 11 for TRPC6−/− cells) or normoxia (black hatched bars; n = 4 each). Cells were primed with ET-1 2 min before treatment. Nifedipine was present throughout the experiments. ∗, P < 0.05 in comparison with normoxia.
Hypoxia Induces DAG Accumulation in PASMC.
To further elucidate signaling steps involved in TRPC6 channel activation, we expressed a fluorescent DAG sensor consisting of the C1 domain of protein kinase C δ (33) fused to the enhanced green fluorescent protein in these cells. In WT and TRPC6−/− PASMC expressing this DAG-sensor a redistribution of fluorescence to the plasma membrane was observed under hypoxic conditions, whereas it was localized in the cytosol in normoxic cells (Fig. 4). Because DAG accumulates at the plasma membrane of WT and TRPC6−/− PASMC after endothelin priming and subsequent hypoxic conditions and exerts its action via activation of TRPC6, our results entertain the notion that TRPC6 is not the primary hypoxia-sensor. Accordingly, recombinant TRPC6 expressed heterologously cannot be activated by hypoxia but by OAG (SI Fig. 11). Furthermore, our findings support the concept that hypoxia-induced TRPC6 activation is mediated by DAG accumulation probably by activated phospholipases. Such an activation may occur via reactive oxygen species (34), which have previously been suggested to trigger HPV (35).
Fig. 4.
Hypoxia-induced DAG accumulation in PASMC. (A) Confocal images before treatment by hypoxia (normoxia) and after treatment by hypoxia (hypoxia) or DAG kinase inhibitor (R59949) of TRPC6−/− and WT PASMC infected with recombinant lentiviruses coding for a fluorescent DAG sensor consisting of the C1 domain of protein kinase C δ (PKCδ) (33) fused to the EGFP. Cells were primed with ET-1 1 min before treatment and were fixed by ice-cold acetone 5 min after treatment. Arrows indicate DAG accumulation at the plasma membrane. (B) Summarized data for the ratios of plasma membrane versus cytosolic fluorescence intensities before treatment (black bars) and after treatment of WT and TRPC6−/− PASMC with hypoxia (gray bars) or DAG kinase inhibitor (gray hatched bars) (n = 37 cells for each group). Data were generated by calculating the fluorescence intensities in regions of interest defined over the plasma membrane and the cytosol of optical slices monitored by confocal microscopy.
To summarize, TRPC6 plays a hitherto unrecognized, indispensable role in acute hypoxic pulmonary vasoconstriction, demonstrating the complete absence of acute HPV by deletion of a single ion channel. Therefore, manipulation of TRPC6 function offers a promising therapeutic strategy for the control of pulmonary hemodynamics and gas exchange.
Materials and Methods
Animals.
All animal experiments were approved by the local authorities. TRPC6−/− mice were generated as described (14). Only 129SV/C57BL/6J littermates (WT or TRPC6−/−) were analyzed in in vivo experiments. Mice of either sex were studied at 2–3 months of age.
Hemodynamic Measurements in Isolated, Perfused, and Ventilated Lungs and in Anesthetized Mice.
Lungs were removed from the chest in deep anesthesia, artificially ventilated, and perfused blood-free as described (refs. 1 and 20 and SI Materials and Methods). For arterial PO2 measurement and airway fluid load, see SI Materials and Methods.
Chronic Hypoxia Exposure, Vascular Remodeling, and Right Ventricular Hypertrophy.
For exposure to chronic hypoxia, mice were kept under normobaric hypoxia [inspiratory O2 fraction (FiO2) 0.10] in a ventilated chamber for 21 days as described (1). The degree of muscularization of small pulmonary arteries and right ventricular hypertrophy was determined as described (1).
SMC Isolation and Culture.
SMCs were isolated from precapillary pulmonary arterial vessels of mice (WT and TRPC6−/−) modified from a previously reported protocol (22). Primary cells were cultured for 3–5 days without passaging. SMCs were identified by immunohistochemical staining with SMC-specific α-actin and myosin antibodies (SI Fig. 7 A and B). The absence of endothelial cells was confirmed by staining with an antibody directed against von-Willebrand factor (SI Fig. 7C). For quantitative RT-PCR analysis, see SI Materials and Methods.
[Ca2+]i Imaging and Mn2+ Quench Experiments in PASMC.
PASMC from WT or TRPC6−/− mice harvested from at least three independent preparations of mice (WT or TRPC6−/−) were analyzed. PASMC on coverslips were loaded with fura-2 acetoxymethyl ester (5 μM) in Hepes-Ringer buffer (HRB; 136.4 mM NaCl/5.6 mM KCl/1 mM MgCl2/2.2 mM CaCl2/10 mM Hepes/5 mM glucose/0.1% BSA, pH 7.4) at 37°C for at least 60 min. Coverslips were then placed on the microscope in a low-volume glass-covered recording chamber, perfused (0.5 ml/min) with HRB (without BSA) saturated with 21% O2 (normoxia, pO2 ≈ 150 mmHg), and maintained at 32 ± 0.2°C by heating both the HRB and the chamber. Cells were primed for hypoxia as described (26) by using ET-1 (4 nM) before the onset of hypoxia. Hypoxia was induced by switching the perfusing medium from normoxia to hypoxia (gassed with N2, resulting in a pO2 of ≈18 mmHg). Drugs were diluted in HRB in separate reservoirs connected to a manifold. PASMCs were analyzed by using a Polychrome II monochromator and an IMAGO CCD camera (Till Photonics, Martinsried, Germany) coupled to an inverted microscope (IX70; Olympus, Hamburg, Germany) at 340 and 380 nm for measuring [Ca2+]i. Mn2+ quench experiments were done as described (36).
Construction of AAVs Coding for TRPC6-EGFP Infection of TRPC6−/− PASMC.
The cDNA coding for mTRPC6-EGFP was subcloned downstream of the simian virus 40 (SV40) promoter and upstream of the SV40 poly(A) sequence into the vector pGL3 (Promega, Mannheim, Germany) replacing the luciferase coding region. The pSV40-mTRPC6-EGFP-SV40polyA fragment was then subcloned into the pSUB201 vector containing only the cis-acting terminal repeats of AAV cloned in a plasmid backbone. The resulting pAAVmTRPC6-EGFP vector and the pDP1 plasmid (containing the rep-2, the cap-1 genes, and the adenoviral VA, E2A, and E4 genes) (37) were cotransfected into HEK293 cells by calcium phosphate precipitation as described (37). Recombinant viruses (rAAV1mTRPC6-EGFP) were purified by CsCl gradient centrifugation, and precapillary pulmonary SMCs were infected at least 3 days before [Ca2+]i imaging analysis.
Construction of Lentiviruses Expressing a Fluorescent DAG-Sensor Molecule.
The C1 fragment of protein kinase Cδ (PKCδ) (33) was cloned into the N1-EGFP vector (Becton Dickinson, Heidelberg, Germany) by using the BglII and Acc65I restriction sites. The reading frame was corrected by fill-up and religation at the Acc65I-site. The PKCδC1-EGFP fragment was excised with BglII and NotI and subcloned into pWPXL. Virus was produced as described earlier (38).
Electrophysiology.
To investigate changes in macroscopic cationic currents caused by hypoxia in PASMCs, the whole-cell patch-clamp technique was used as described (ref. 14 and SI Materials and Methods).
Statistics.
Data were assessed by using ANOVA with the Student–Newman–Keuls post hoc test or Student's t test with Welsh's correction as appropriate. Data are expressed as mean ± SEM. Intergroup differences of changes of the effects of drugs on whole-cell current (Fig. 3) were assessed by a factorial ANOVA with post hoc analysis with Fisher's least significant difference test. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Paul T. Schumacker (Northwestern University, Chicago, IL) for providing a protocol for PASMC isolation and Lutz Birnbaumer (National Institute on Enviornmental Health Sciences, Research Triangle Park, NC) for continous support; Silke Kaske for help in confocal microscopy; J. A. Kleinschmidt (German Cancer Research Center, Heidelberg, Germany) for the gift of DP plasmids; T. Meyer (Stanford University, Stanford, CA) for the gift of the plasmid coding for the fluorescent DAG sensor; and Carmen Homberger, Karin Quanz, Winfried Lorenz, and Susanne Ziegler for excellent technical assistance. This work was funded by Deutsche Forschungsgemeinschaft Grant SFB547, Project B7 (NW), and a grant to A.D. and T.G.
Abbreviations
- [Ca2+]i,
intracellular Ca2+ concentration
- DAG
diacylglycerol
- HPV
hypoxic pulmonary vasoconstriction
- SMC
smooth-muscle cell
- PASMC
precapillary pulmonary arterial SMC
- TRPC
transient receptor potential cation channel subfamily C
- ET-1
endothelin-1
- OAG
membrane-permeable analogue of DAG
- HRB
Hepes-Ringer buffer
- AAV
adeno-associated virus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606728103/DC1.
References
- 1.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffery TK, Wanstall JC. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 3.Weissmann N, Winterhalder S, Nollen M, Voswinckel R, Quanz K, Ghofrani HA, Schermuly RT, Seeger W, Grimminger F. Am J Physiol. 2001;280:L638–L645. doi: 10.1152/ajplung.2001.280.4.L638. [DOI] [PubMed] [Google Scholar]
- 4.Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Lancet. 1999;353:2205–2207. doi: 10.1016/S0140-6736(98)08352-4. [DOI] [PubMed] [Google Scholar]
- 5.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Am J Physiol. 2005;289:L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Am J Physiol. 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- 7.McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Am J Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- 8.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 9.Montell C. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich A, Kalwa H, Rost BR, Gudermann T. Pflügers Arch. 2005;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Pharmacol Ther. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 13.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan J X. Proc Natl Acad Sci USA. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waypa GB, Schumacker PT. J Appl Physiol. 2005;98:404–414. doi: 10.1152/japplphysiol.00722.2004. [DOI] [PubMed] [Google Scholar]
- 17.Weissmann N, Grimminger F, Olschewski A, Seeger W. Am J Physiol. 2001;281:L314–L317. doi: 10.1152/ajplung.2001.281.2.L314. [DOI] [PubMed] [Google Scholar]
- 18.Wolin MS, Ahmad M, Gupte SA. Am J Physiol. 2005;289:L159–L173. doi: 10.1152/ajplung.00060.2005. [DOI] [PubMed] [Google Scholar]
- 19.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissmann N, Akkayagil E, Quanz K, Schermuly RT, Ghofrani HA, Fink L, Hanze J, Rose F, Seeger W, Grimminger F. Respir Physiol Neurobiol. 2004;139:191–202. doi: 10.1016/j.resp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 22.Waypa GB, Chandel NS, Schumacker PT. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 23.Sham JSK, Crenshaw BR, Jr, Deng L-H, Shimoda LA, Sylvester JT. Am J Physiol. 2000;279:L262–L272. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- 24.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JPT. J Physiol (London) 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimoda LA, Sham JSK, Liu Q, Sylvester JT. Respir Physiol Neurobiol. 2002;132:93–106. doi: 10.1016/s1569-9048(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 26.Ward JP, Robertson TP. Exp Physiol. 1995;80:793–801. doi: 10.1113/expphysiol.1995.sp003887. [DOI] [PubMed] [Google Scholar]
- 27.Ward JP, Robertson TP, Aaronson PI. Am J Physiol. 2005;289:L2–L4. doi: 10.1152/ajplung.00101.2005. [DOI] [PubMed] [Google Scholar]
- 28.Dipp M, Evans M. Circ Res. 2001;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- 29.Estacion M, Sinkins WG, Jones SW, Applegate MA, Schilling WP. J Physiol (London) 2006;572:359–377. doi: 10.1113/jphysiol.2005.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudermann T, Schnitzler M, Dietrich A. Sci STKE. 2004;2004:e35. doi: 10.1126/stke.2432004pe35. [DOI] [PubMed] [Google Scholar]
- 31.Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- 32.French RJ, Wells JB. Gen Physiol. 1977;70:707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S. J Cell Biol. 2000;151:1353–12367. doi: 10.1083/jcb.151.7.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groschner K, Rosker C, Lukas M. Novartis Found Symp. 2004;258:222–230. [PubMed] [Google Scholar]
- 35.Weissmann N, Sommer N, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Cardiovasc Res. 2006;71:620–629. doi: 10.1016/j.cardiores.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. J Biol Chem. 2003;278:47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- 37.Grimm D, Kay MA, Kleinschmidt JA. Mol Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 38.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.