Abstract
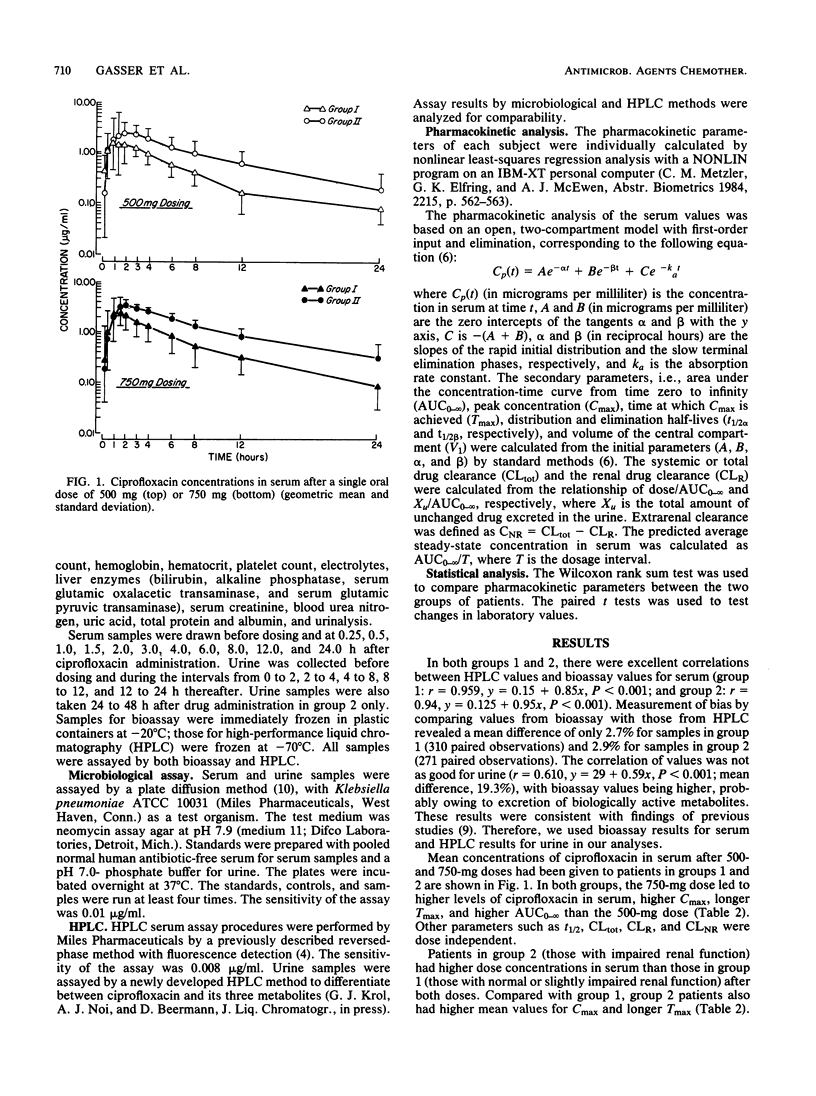
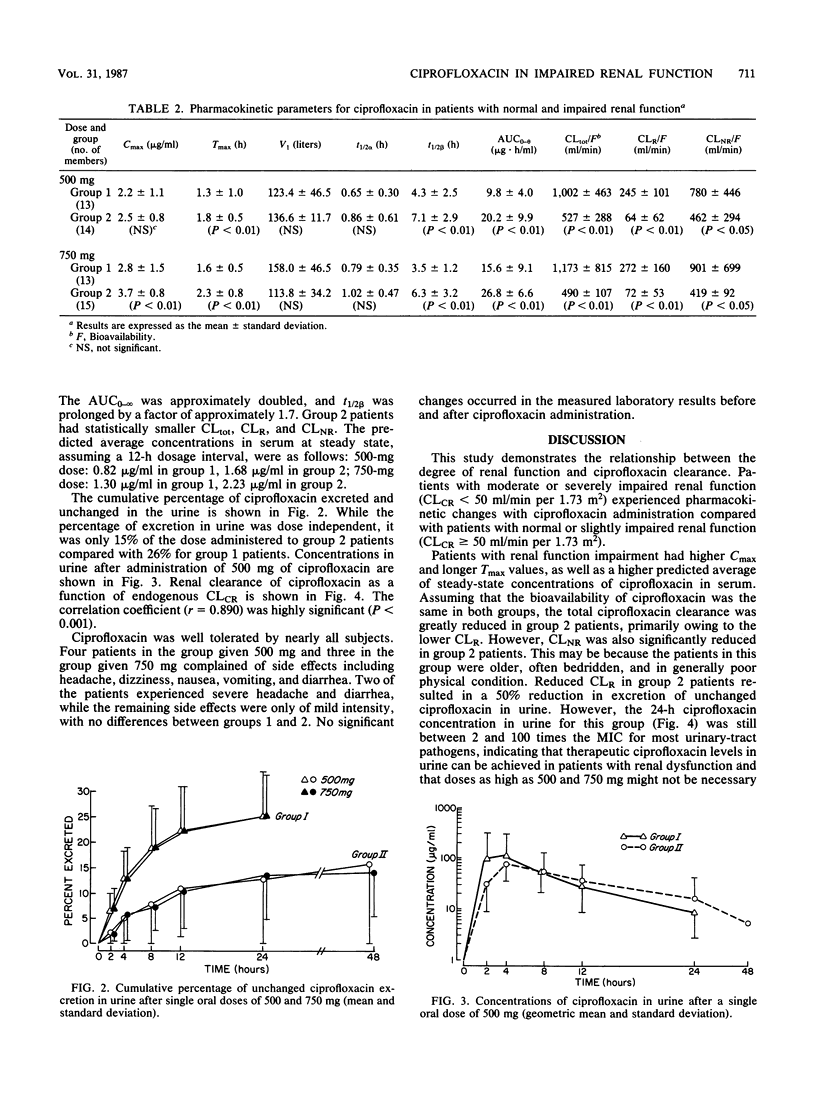
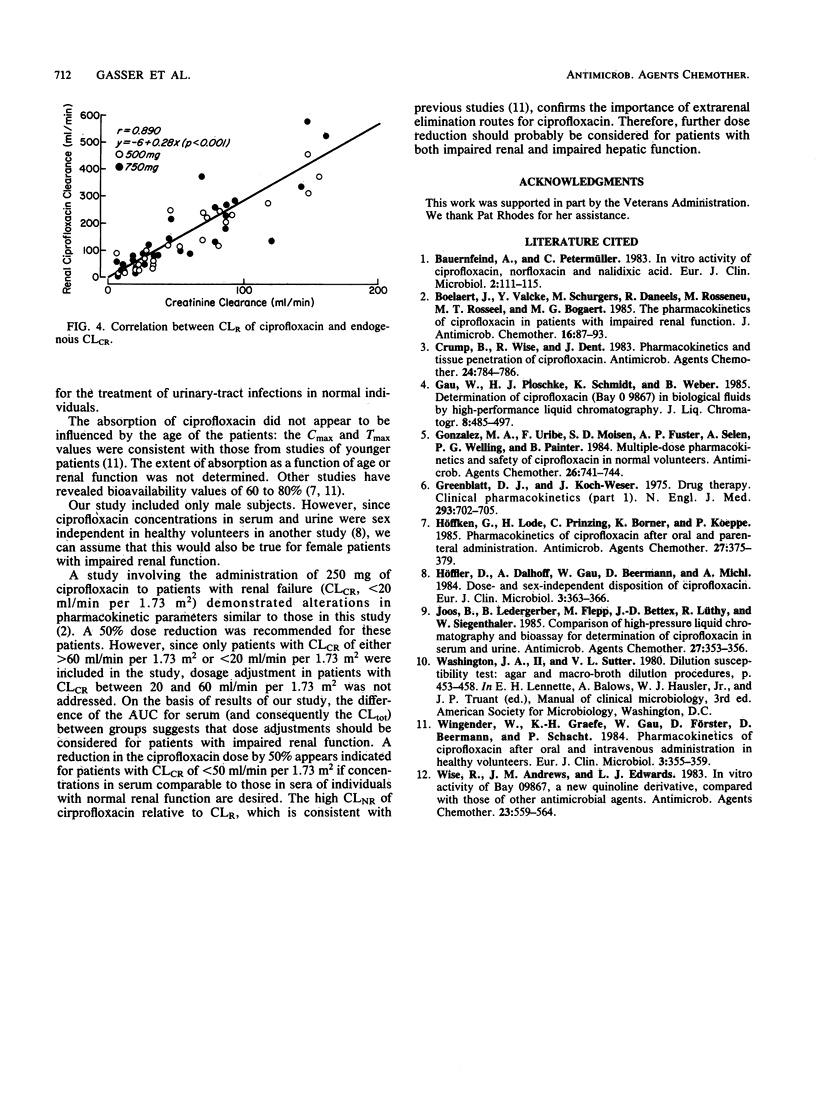
The pharmacokinetics of ciprofloxacin following single oral doses of 500 and 750 mg in 32 patients with various degrees of renal function impairment were investigated in an open, randomized crossover fashion. Ciprofloxacin was administered after overnight fasting; the washout time between the two doses was 1 week. Serum and urine samples were collected serially between 0 and 24 h and subjected to bioassay and high-performance liquid chromatography. Pharmacokinetic parameters were analyzed, assuming an open two-compartment model with first-order input and elimination. A distinct difference was observed in pharmacokinetic parameters between patients with impaired renal function (creatinine clearance, less than 50 ml/min per 1.73 m2) and those with normal renal function (creatinine clearance, greater than or equal to 50 ml/min per 1.73 m2). For the former group, the area under the curve of serum concentration versus time was doubled, the renal clearance of ciprofloxacin was cut to one-fourth, the total and nonrenal ciprofloxacin clearance was reduced by 50%, and the elimination half-life was prolonged by a factor of approximately 1.7. The correlation between renal drug clearance and creatinine clearance was highly significant (r = 0.890; P less than 0.001). On the basis of these findings, it appears that a 50% dose reduction of ciprofloxacin in patients with impaired renal function (creatinine clearance, less than 50 ml/min per 1.73 m2) may be indicated to achieve concentrations in serum similar to those observed in normal individuals. As the concentration of ciprofloxacin in urine after 24 h remained above the MIC for most urinary pathogens, this drug appears to be of potential benefit for the treatment of urinary tract infections in patients with impaired renal function.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Boelaert J., Valcke Y., Schurgers M., Daneels R., Rosseneu M., Rosseel M. T., Bogaert M. G. The pharmacokinetics of ciprofloxacin in patients with impaired renal function. J Antimicrob Chemother. 1985 Jul;16(1):87–93. doi: 10.1093/jac/16.1.87. [DOI] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Kock-Weser J. Drug therapy. Clinical Pharmacokinetics (first of two parts). N Engl J Med. 1975 Oct 2;293(14):702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffler D., Dalhoff A., Gau W., Beermann D., Michl A. Dose- and sex-independent disposition of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):363–366. doi: 10.1007/BF01977496. [DOI] [PubMed] [Google Scholar]
- Joos B., Ledergerber B., Flepp M., Bettex J. D., Lüthy R., Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985 Mar;27(3):353–356. doi: 10.1128/aac.27.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender W., Graefe K. H., Gau W., Förster D., Beermann D., Schacht P. Pharmacokinetics of ciprofloxacin after oral and intravenous administration in healthy volunteers. Eur J Clin Microbiol. 1984 Aug;3(4):355–359. doi: 10.1007/BF01977494. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



