Abstract
Prolonged sleep deprivation is stressful and has been associated with adverse consequences for health and cognitive performance. Here, we show that sleep deprivation inhibits adult neurogenesis at a time when circulating levels of corticosterone are elevated. Moreover, clamping levels of this hormone prevents the sleep deprivation-induced reduction of cell proliferation. The recovery of normal levels of adult neurogenesis after chronic sleep deprivation occurs over a 2-wk period and involves a temporary increase in new neuron formation. This compensatory increase is dissociated from glucocorticoid levels as well as from the restoration of normal sleep patterns. Collectively, these findings suggest that, although sleep deprivation inhibits adult neurogenesis by acting as a stressor, its compensatory aftereffects involve glucocorticoid-independent factors.
Keywords: dentate gyrus, stress, corticosterone, cell proliferation, adrenal steroids
Sleep is important for health and survival. Extended sleep deprivation results in the deterioration of many basic functions (1, 2) and induces physiological changes similar to those seen after repeated stress, including reductions in body temperature and weight, despite increased energy expenditure and hyperphagia (3–6). Moreover, the neuroendocrine changes observed after prolonged sleep deprivation parallel those observed after stress. That is, both increase the release of corticotropin-releasing factor (7) and elevate plasma levels of adrenocorticotropic hormone and corticosterone (CORT; ref. 8–11), as well as progesterone (8, 12), while reducing those of testosterone (8, 13). Many behavioral paradigms have been used to induce sleep deprivation, and these vary in the degree to which their application is stressful. However, extended sleep loss appears to be stressful in itself, regardless of the method used.
Recent reports have suggested that sleep facilitates adult neurogenesis in the hippocampus, based on findings that prolonged sleep deprivation reduces cell proliferation and neurogenesis in the dentate gyrus of adult rats (14–17). However, because glucocorticoids and stress are known to inhibit adult neurogenesis (18), it is possible that sleep deprivation effects on this process stem from its actions as a stressor. Here we show that sleep deprivation-induced decreases in cell proliferation are the result of elevated glucocorticoid levels. Moreover, we examine the long-term consequences of sleep deprivation on adult neurogenesis during the recovery period and report a delayed enhancement of this phenomenon, which appears to be dissociated from glucocorticoids as well as from the restoration of normal sleep patterns.
Results
The small-platform (SP) method is a well established protocol for inducing sleep deprivation (19–21), in which large-platform (LP) exposure and nondeprived animals serve as controls. As reported (19, 20), telemetry-implanted animals subjected to the SP condition had reduced overall sleep and almost complete elimination of time spent in rapid-eye-movement sleep (REM). In addition, nonimplanted SP animals revealed a characteristic reduction in body weight after 72 h of sleep deprivation (F1,13 = 20.60, P = 0.001), compared with cage controls (CC), consistent with another report (21). After recovery from sleep deprivation, SP-exposed animals resumed normal rates of weight gain within 3 days and continued to do so after 1 wk.
Sleep Deprivation-Induced Decreases in Neurogenesis Coincide with Elevated Plasma CORT Levels.
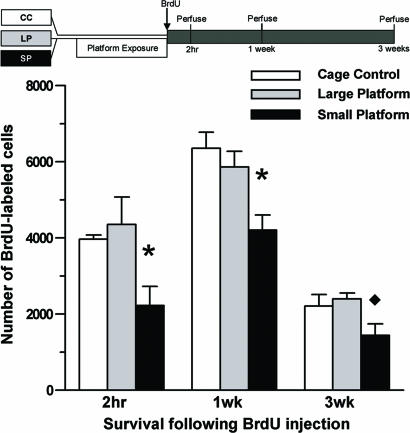
To examine whether acute (24-h) or prolonged (72-h) sleep loss affects cell proliferation and adult neurogenesis, BrdU-labeled cell counts were determined in the granule cell layer (GCL) of adult animals after SP, LP, or CC conditions. Two hours after BrdU administration, the number of BrdU-labeled cells of animals subjected to 24 h of sleep deprivation did not differ from either control group (F2,18 = 0.61, P = 0.552). However, the number of BrdU-labeled cells was significantly reduced in animals subjected to 72 h of sleep deprivation compared with both control groups (CC and LP animals; F2,11 = 5.93, P = 0.018; Fig. 1). This difference persisted to the 1-wk survival time after BrdU injection, because the 72-h SP animals had fewer BrdU-labeled cells in the dentate gyrus than the CC and LP animals (F2,15 = 7.26, P = 0.006; Fig. 1). At this time, the majority of BrdU-labeled cells were also positive for class III β-tubulin (TuJ1; ≈70%), a marker of immature and mature neurons (Fig. 2). No differences in the proportion of BrdU-labeled cells expressing this marker were noted across groups. By 3 wk after BrdU administration, when the majority of BrdU-labeled cells expressed the mature neuronal marker neuronal nuclei (NeuN; ≈80%; Fig. 2), decreased BrdU cell counts in the 72-h SP animals persisted (F2,13 = 4.24, P = 0.038; Fig. 1). In contrast to the GCL, no effect of sleep deprivation on the density of BrdU cell counts was observed in the subventricular zone (SVZ; F2,15 = 2.06, P = 0.14; CC, 17,446 ± 3,198 cells/mm3; LP, 15,710 ± 1,114 cells/mm3; SP, 15,430 ± 2,970 cells/mm3) 2 h after BrdU administration, suggesting that sleep deprivation reduces cell proliferation with some regional specificity.
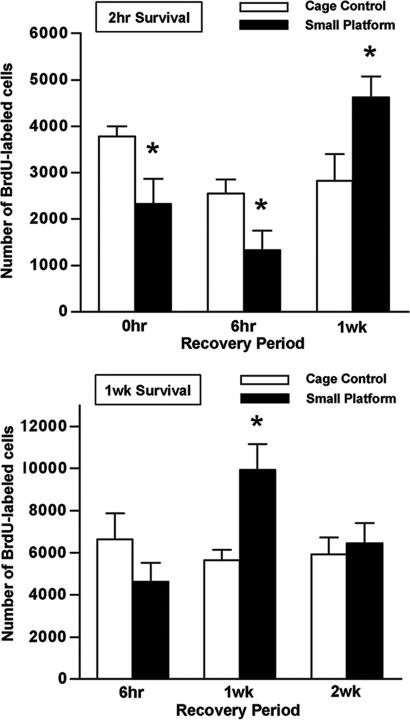
Fig. 1.
Reduced cell proliferation and adult neurogenesis after prolonged SP exposure. Rats subjected to 72 h of SP, LP, or CC received a single injection of BrdU (200 mg/kg, i.p.) and were perfused 2 h, 1 wk, or 3 wk thereafter. Compared with CC and LP rats, SP rats had fewer numbers of BrdU-positive cells in the subgranular zone/GCL at 2 h (SP, n = 5; LP, n = 4; CC, n = 5). At 1 wk, significantly lower numbers of BrdU-positive cells were found in SP (n = 6), relative to CC (n = 7) and LP (n = 5) rats. By 3 wk, fewer BrdU-labeled cells were evident after SP exposure (n = 6), compared with LP (n = 6) but not CC (n = 4) rats. Error bars indicate the SEM; ∗, P < 0.05, SP vs. CC, LP; ♦, P < 0.05, SP vs. LP.
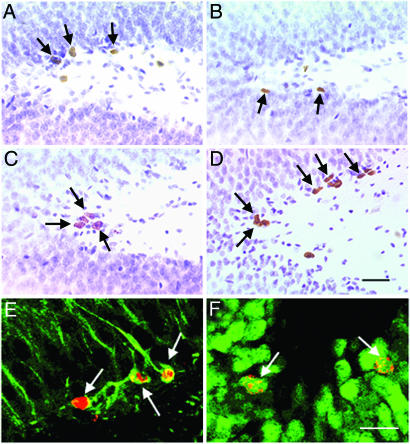
Fig. 2.
Sleep deprivation-induced reduction and subsequent increase in cell proliferation and adult neurogenesis. (A and B) Compared with CC rats (A), stereological estimates of BrdU-labeled cells in the GCL revealed a reduction in cell counts in SP rats (B) 2 h after BrdU administration. (C and D) In contrast, after 1 wk of unrestricted recovery from prolonged sleep deprivation, BrdU cell counts in SP rats (D) were significantly increased compared with CC rats (C). (E and F) By 1 wk after BrdU administration, the majority of BrdU-positive cells showed morphological characteristics of granule cells and were colabeled with TuJ1 (E), a marker of immature and mature neurons. By 3 wk after BrdU administration, most BrdU-positive cells were also colabeled with NeuN (F), a marker of mature neuronal phenotype. (Scale bars: A–D, 40 μm; E and F, 20 μm.)
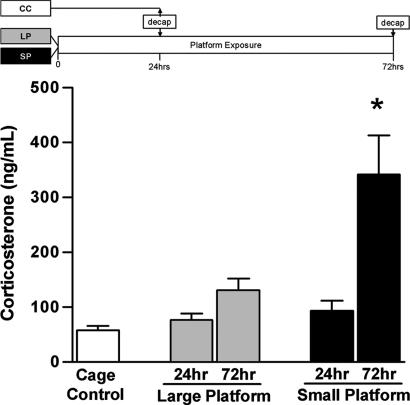
Separate groups of animals were examined under SP, LP, and CC conditions for determination of CORT levels. No differences were observed between animals deprived of sleep for 24 h and controls. However, 72 h of SP exposure resulted in a significant increase in this measure compared with the other groups (F4,27 = 12.52, P < 0.001; Fig. 3). Thus, CORT levels increase after a duration of sleep deprivation that is associated with a decrease in adult neurogenesis.
Fig. 3.
Elevated CORT levels after prolonged SP exposure. To identify whether changes in CORT levels emerge after acute or prolonged sleep deprivation, blood was collected from animals exposed to LP or SP for 24 h (LP24 h, n = 6; SP24 h, n = 6) and 72 h (LP72 h, n = 6; SP72 h, n = 6), as well as from CC animals (n = 8). Compared with all other groups, animals subjected to 72 h of SP exposure exhibited increased levels of CORT. Error bars indicate SEM; ∗, P < 0.05 compared with control.
Sleep Deprivation-Induced Suppression of Cell Proliferation Requires Elevated Glucocorticoids.
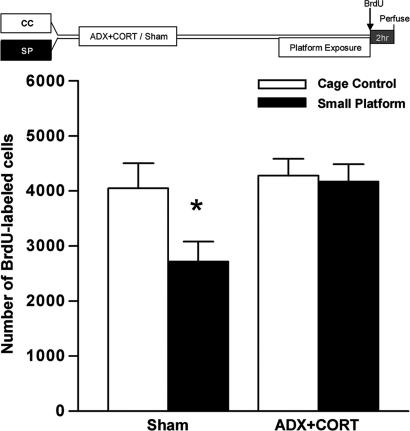
To determine whether CORT elevation is responsible for suppressing cell proliferation after extended sleep deprivation, we examined the effects of 72 h of SP exposure in sham-operated (Sham) or adrenalectomized (ADX) animals receiving low-dose CORT (ADX+CORT) in the drinking water on the number of BrdU-labeled cells in the dentate gyrus after a 2-h survival time. Sham animals exposed to extended sleep deprivation demonstrated the characteristic reduction in BrdU-labeled cells; this effect was completely eliminated in ADX+CORT animals (F1,17 = 1.792, P = 0.198; Fig. 4). These results suggest that elevated glucocorticoids are required for the reduction in cell proliferation associated with prolonged sleep deprivation. No differences in CORT levels were noted among control (22.6 ± 1.20 ng/ml) or SP-exposed (27.2 ± 4.49 ng/ml) ADX animals, validating the efficacy of glucocorticoid normalization by this method and dose of hormone replacement.
Fig. 4.
Prevention of sleep deprivation-associated reduction of cell proliferation by CORT normalization. Adult rats underwent bilateral surgical removal of the adrenal glands (ADX) or were Sham-operated. All ADX-treated rats were given drinking water containing CORT (25 μg/ml in 0.9% saline). One week after surgery, ADX and Sham rats either remained as controls (Sham/CC, n = 5; ADX+CORT/CC, n = 5) or were exposed to 72 h on SPs (Sham/SP, n = 7; ADX+CORT/SP, n = 4). Thereafter, all rats received a single injection of BrdU (200 mg/kg, i.p.) and were perfused 2 h later. The suppression of cell proliferation normally associated with extended sleep loss was observed in Sham/SP but not ADX+CORT/SP rats; that is, fewer BrdU-labeled cells were observed in Sham/SP rats compared with all other groups. Error bars indicate SEM; ∗, P < 0.05 compared with control.
Recovery and Overshoot of Cell Proliferation After Sleep Deprivation.
To determine whether and when the sleep deprivation-induced decrease in cell proliferation and neurogenesis returns to baseline levels, animals were allowed to recover from prolonged sleep deprivation for 6 h, 1 wk, or 2 wk before BrdU administration. BrdU cell counts in the dentate gyrus were examined at the 2-h or 1-wk survival time points and compared with CC animals. After 6 h of unrestricted sleep, a time characterized by excess sleep as well as REM rebound (22), fewer BrdU-labeled cells were found in SP animals at the 2-h but not 1-wk survival period (Fig. 5). In contrast, after 1 wk of recovery from sleep deprivation, an overshoot in the number of BrdU-labeled cells was observed after 2 h (F2,24 = 8.325, P = 0.002) and 1 wk after BrdU injection (F2,27 = 5.206, P = 0.012). However, 2 wk after recovery from prolonged sleep deprivation, the number of BrdU-labeled cells appeared to return to control levels. These data suggest that extended sleep deprivation is also associated with a delayed temporary increase in adult neurogenesis, an effect that appears to be temporally dissociated from the recovery of normal sleep patterns.
Fig. 5.
Lasting effects of prolonged sleep deprivation on cell proliferation and adult neurogenesis. Rats that were either subjected to 72 h of sleep deprivation by SP exposure (SP) or maintained as controls (CC) received a single injection of BrdU (200 mg/kg, i.p.) 6 h, 1 wk, or 2 wk later. Rats were then perfused at the 2-h (Upper) or 1-wk (Lower) survival periods to examine changes in cell proliferation and adult neurogenesis, respectively (n = 4–8). SP rats exhibited reduced cell proliferation but no effect on adult neurogenesis 6 h after unrestricted sleep recovery. In contrast, after 1 wk of recovery, both cell proliferation and adult neurogenesis were enhanced in SP animals. By 2 wk of recovery, no difference in neurogenesis was observed. Error bars indicate SEM; ∗, P < 0.05 compared with control. For purposes of comparison, BrdU-labeled cell counts for the 0-h recovery time from Fig. 1 are also presented here.
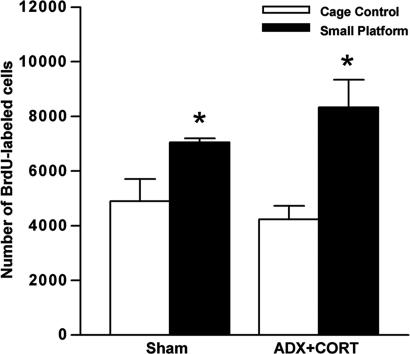
Temporary Overshoot of Cell Proliferation After Sleep Deprivation Does Not Require Elevated Glucocorticoids.
To determine whether glucocorticoids are involved in the increase in cell proliferation that occurs after sleep deprivation, we examined the number of BrdU-labeled cells in ADX+CORT and Sham animals after sleep deprivation followed by a 1-wk recovery period. As expected, Sham animals displayed an increase of BrdU-labeled cells at the 2-h post-BrdU injection time point, compared with both control groups (Fig. 6). This effect was not altered in the ADX+CORT animals; those subjected to prolonged sleep deprivation also exhibited increased BrdU cell counts after 1 wk of recovery compared with both control groups (F1,17 = 1.679, P = 0.212; Fig. 6). These findings suggest that the overshoot in cell proliferation is independent of elevated glucocorticoids and, furthermore, occurs even in the absence of a sleep deprivation-induced inhibition of cell proliferation.
Fig. 6.
Sustained overshoot of cell proliferation after sleep deprivation despite CORT normalization. Adult rats underwent bilateral surgical removal of the adrenal glands (ADX) or were Sham-operated. All ADX-treated rats were given drinking water containing CORT (25 μg/ml in 0.9% saline). One week after surgery, ADX and Sham rats either remained as controls (Sham/CC, n = 4; ADX+CORT/CC, n = 6) or were exposed to 72 h on SPs (Sham/SP, n = 4; ADX+CORT/SP, n = 6). One week later, all rats received a single injection of BrdU (200 mg/kg, i.p.) and were perfused 2 h later. A delayed enhancement of cell proliferation after extended sleep loss was observed in both Sham and ADX+CORT animals. Error bars indicate SEM; ∗, P < 0.05.
Discussion
These results suggest that sleep deprivation reduces cell proliferation and adult neurogenesis in the dentate gyrus by elevations in glucocorticoids. Although 24 h of sleep deprivation affected neither cell proliferation nor CORT levels, 72 h of sleep deprivation substantially lowered cell proliferation and increased stress hormone levels. Moreover, preventing the elevation of glucocorticoids in animals sleep-deprived for 72 h completely eliminated the decrease in cell proliferation. Our results also demonstrate a recovery and delayed overshoot of adult neurogenesis after sleep deprivation, which is temporally dissociated from rebound sleep. Six hours after prolonged sleep deprivation, a time characterized by maximal REM rebound (22), cell proliferation remained inhibited. But 1 wk later, when animals have resumed normal sleep patterns (21, 23), the production of new cells exceeds that of controls. This overshoot in cell proliferation does not seem linked to glucocorticoids, because clamping CORT levels throughout the sleep deprivation and recovery period does not eliminate this effect.
The reduction of cell proliferation and adult neurogenesis has also been shown in previous studies by using different methods of sleep deprivation, such as the treadmill and disk-over-water methods (14–17). With the disk-over-water paradigm, animals are motivated to remain awake, because continued sleep runs the risk of falling into water; animals can escape this consequence if they move with the rotating disk. In contrast, animals subjected to the treadmill method cannot avoid being disturbed by maintaining wakefulness, because treadmill movement is not tethered to behavioral state but is rather periodic and thus forced and unrelated to sleep (17). Although each of these methods of sleep deprivation presents animals with different consequences and degrees of predictability, all are inherently disruptive and likely stressful, particularly if applied chronically.
A previous study examined the relationship between glucocorticoid levels and reduced cell proliferation with sleep deprivation (using a forced locomotion paradigm) and found no connection. However, in that study, CORT was assessed at a different time point, i.e., after 48 h of sleep deprivation (17), from when cell proliferation was examined. Moreover, running has been shown to affect CORT levels but only at the diurnal peak of stress hormone secretion (24, 25). Thus, elevated glucocorticoids may be missed when blood samples are obtained only at single time points after sleep deprivation. Taken together with our results, this finding suggests that a temporal relationship between elevated CORT and reduced cell proliferation with sleep deprivation may emerge some time after 48 h.
It is unlikely that our results are specific to the platform method of sleep deprivation, because the suppressive effects on cell proliferation were completely eliminated when glucocorticoids were kept constant. This suggests that sleep deprivation alone, even with the platform method, is ineffective at reducing cell production in the absence of elevated stress hormones. Rather, the elevation of CORT appears to be necessary for the inhibition of adult neurogenesis and determines the timing of this effect. Thus, our findings are consistent with previous studies demonstrating inhibition of adult neurogenesis using more traditional stressors (18). The underlying pathways of glucocorticoid-induced suppression of cell proliferation may involve both direct effects through glucocorticoid receptors (18) and indirect effects through NMDA receptor signaling, because neural progenitor cells are known to express both of these receptors (26, 27). However, a detailed characterization of glucocorticoid signaling in progenitor cells of the dentate gyrus has not yet been carried out.
One possible alternative interpretation of the present and prior findings (14–17) may be that sleep itself is a permissive time for cell proliferation and neurogenesis, because it coincides with low glucocorticoid levels. Typically, sleep occurs during periods of the diurnal rhythm when CORT levels are reduced (28, 29). However, several lines of evidence argue against this possibility. First, no previously published evidence suggests that more cell production occurs during sleep than during wakefulness, nor has a diurnal rhythm in the number of proliferating cells been reported in the subgranular zone/GCL of rodents (30, 31) under standard control conditions. Second, the period immediately after prolonged sleep deprivation, which is characterized by an overshoot in the amount of sleep, is not temporally associated with an increase in cell proliferation. Instead, the period of sleep rebound coincides with a continued suppression of cell proliferation (14), which resembles the pattern observed after acute stress exposure (32). Furthermore, previous studies that have experimentally reduced CORT levels to the low physiological range have not reported increases in cell proliferation (33, 34). Thus, sleep and adult neurogenesis appear related only during conditions of extended sleep loss, when the deprivation period is sufficient to elevate glucocorticoids.
A rebound in cell proliferation and adult neurogenesis was eventually observed after prolonged sleep deprivation, but this did not emerge until considerably after the time when normal sleep patterns reestablish (23). In contrast to the glucocorticoid-mediated suppression of cell proliferation, the increase in cell proliferation and neurogenesis observed 1 wk later persisted despite normalized CORT levels. Thus, the subsequent overshoot in new neurons did not depend on a prior decrease in cell proliferation. Because the underlying factors of this protracted increase in adult neurogenesis remain to be explored, it is presently unknown whether this is due to nonglucocorticoid stress effects or is instead attributable to extended sleep deprivation itself. Common targets of both stress and sleep deprivation include various growth factors (35–38). Adult neurogenesis is known to be regulated by such extracellular signals (39), although the potential protracted effects of stress or sleep deprivation on neurotrophic levels have not been well characterized.
Although the functional significance of adult neurogenesis remains unknown, the suppression of adult neurogenesis may underlie some of the cognitive deficits associated with prolonged sleep deprivation (39). Likewise, the overshoot and recovery of normal levels of adult neurogenesis after sleep deprivation is over may be related to the restoration of normal cognitive function, because adult-generated neurons have been suggested to play an important role in certain types of learning and memory (40, 41). Although adult neurogenesis may be important for specific types of learning, it is also possible that sleep deprivation has detrimental effects on other forms of structural plasticity, because glucocorticoids are also known to induce atrophy of dendrites in other regions of the hippocampus (42). Thus, although sleep is undoubtedly critical for health (1, 2) and may be important for cognitive function (43), the present evidence does not suggest that sleep itself promotes adult neurogenesis, but rather that the stressful nature of sleep deprivation exerts negative effects on the hippocampus.
Materials and Methods
Animal Treatments.
Adult male Sprague–Dawley rats (225–350 g) were housed two to three per cage with ad libitum access to food and water and maintained on a 12/12-h light/dark cycle (lights on at 7:00 a.m.). All animal experimentation was conducted in accordance with Princeton University guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sleep Deprivation Procedures.
Rats were divided into one of three experimental groups: SP, LP, or CC. SP rats were placed individually onto a circular metal platform (diameter, 6 cm). The platform was located in an opaque plastic container filled with room-temperature water, 10 cm above the water line. During sleep, REM-associated muscle atonia caused animals to fall into the water, forcing them to climb back on the platform and remain awake. LP rats were placed in an identical apparatus but equipped with a larger platform (diameter, 20 cm) to permit sleep, serving as a control for the general environmental conditions of the SP. Additionally, CC rats were placed individually onto clean bedding in standard home cages. All animals were tested in similar temperature- and humidity-controlled colonies, and all platform exposures began at 2:00 p.m. and ended after 24 or 72 h, to evaluate differences in acute vs. extended sleep deprivation, respectively. In the case of sleep-recovery experiments, CC animals were transferred to new home cages at the time of SP exposure cessation.
Electroencephalographic (EEG) Recordings.
The efficacy of the disk-over-water method to induce sleep deprivation has been well described (19–21). Because both stress and brain damage have been shown to alter adult neurogenesis (18, 44), we did not obtain EEG recordings from the animals used in our BrdU or CORT experiments. Instead, we verified the efficacy of this method in a small subset of animals (n = 2) implanted with telemetry from Data Sciences International (St. Paul, MN). These devices allow for continuous remote monitoring of EEG and electromyographic (EMG) data. Rats were deeply anesthetized (ketamine/xylazine; 75/15 mg/kg, i.p.) and secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Transmitter lead wires for EEG recordings were placed in three burr holes (one over the frontal sinus; others placed +2 mm anterior–posterior and ±2 mm medial–lateral from lambda) and sealed with dental acrylic. EMG wires were implanted into neck muscle. The transmitter and electrode wires were placed s.c. in the loose skin of the dorsal neck and back. Animals were allowed to recover from surgery for at least 1 wk before continuous recording. Baseline data were collected for 3 days, after which the animals were placed on the SPs for 72 h. Rats were then individually housed in home cages for 1 additional week after sleep deprivation. To allow for comparison of percent time across behavioral states (waking, W; slow-wave sleep, SW; REM), 1-h segments of continuous EEG/EMG records were analyzed by using Dataquest Art software (Transoma Medical, Arden Hills, MN).
Animals used for the CORT and BrdU studies were observed behaviorally to assess whether they were asleep or awake, and body weight was recorded before and immediately after prolonged sleep deprivation, as well as after 3 days and 1 wk of recovery. Despite increased food intake (21), animals are known to exhibit arrested weight gains during prolonged sleep deprivation.
BrdU Labeling After Sleep Deprivation and Recovery.
To determine whether acute (24-h) or chronic (72-h) sleep loss affects cell proliferation and adult neurogenesis, animals from each group (SP, LP, and CC) were given a single injection of BrdU (200 mg/kg body weight, i.p., in saline, 0.007 M NaOH; Sigma, Milwaukee, WI) at ≈2:00 p.m. These animals were perfused with 4.0% paraformaldehyde in 0.1 M phosphate buffer at 2-h, 1-wk, or 3-wk survival times. The 2-h timepoint was selected because it is sufficient for BrdU uptake but not mitosis or differentiation (45). The 1-wk timepoint was selected because at this time, most BrdU-labeled cells in the dentate gyrus express markers of immature neurons, such as TuJ1 (34, 45). By the 3-wk timepoint, the majority of BrdU-labeled cells express markers of mature neurons, such as NeuN (34).
To examine the recovery of cell proliferation and neurogenesis after sleep deprivation, additional rats were subjected to either SP or CC conditions for 72 h and then returned to standard laboratory cages and allowed to recover. The LP group was not included in this study, because the results of the previous experiment showed no statistical differences compared with caged controls. These animals were perfused at 6 h, 1 wk, or 2 wk after sleep deprivation was terminated. The 6-h recovery period corresponds to the time when animals spend a disproportionate amount of time in sleep after prolonged deprivation (22). However, by 1 wk, changes in sleep and body weight have returned to normal (21, 23). After recovery periods of varying lengths, animals were injected with BrdU and perfused either 2 h or 1 wk after BrdU injection, to examine changes in cell proliferation and immature neuron production.
Glucocorticoid Measurements.
To assess whether sleep deprivation alters glucocorticoid levels, additional animals were exposed to SP or LP conditions for 24 or 72 h, whereas CC animals served as controls. All animals were rapidly decapitated at ≈2:00 p.m. (the time of BrdU injections in the present study), and trunk blood was collected. For analysis, CORT levels were determined by using a radioimmunoassay kit (detection limit, 5.7 ng/ml; cross-reactivity, <3.0% for deoxycorticosterone and <1.0% for all other steroid hormones; Coat-A-Count kit; Diagnostic Products, Los Angeles, CA).
Glucocorticoid Manipulation.
To determine whether the reduction in cell proliferation after prolonged sleep deprivation is mediated by CORT, adult rats were either bilaterally ADX or Sham under deep sodium pentobarbital anesthesia (40 mg/kg, i.p.). To maintain plasma electrolytes and prevent granule cell death associated with complete CORT removal (45), ADX animals were given a low dose of CORT in drinking water (25 μg/ml in 0.9% NaCl; Sigma). One week after surgery, animals were subjected to either prolonged SP exposure or CC conditions. Either immediately after prolonged sleep deprivation (72 h) or after 1 additional week of recovery from sleep restriction, animals were injected with BrdU and then perfused 2 h later, to assess changes in cell proliferation. To verify the efficacy of adrenalectomy, trunk blood was collected during perfusion. CORT levels were determined as described.
Histology.
Brains were postfixed in 4.0% paraformaldehyde in 0.1 M PBS. An oscillating tissue slicer was used to cut 40-μm unilateral coronal sections through the dentate gyrus. For peroxidase BrdU-immunolabeling, sections were mounted onto slides, incubated in 0.1 M citric acid (pH 6.0) at 90°C for 20 min, rinsed in PBS (as in all steps), digested by using trypsin (0.001% in Tris buffer containing 0.1% CaCl2) for 10 min, rinsed, denatured in 2 M HCl for 30 min, rinsed, and incubated in mouse anti-BrdU (1:200 + 0.5% Tween 20; Novocastra, Newcastle Upon Tyne, U.K.) at 4°C overnight. The following day, sections were rinsed, incubated in biotinylated mouse secondary antisera (1:200; Vector Laboratories, Burlingame, CA) for 60 min, rinsed, incubated in avidin-biotin-horseradish peroxidase (1:100; Vector Laboratories) for 60 min, rinsed, and reacted in 0.01% diaminobenzidine with 0.003% H2O2 in distilled water for 8 min. The slides were counterstained by using 0.5% cresyl violet, dehydrated in graded ethanols, cleared with Citrisolv (Fisher Scientific, Pittsburgh, PA), and cover-slipped under Permount (Fisher Scientific).
For immunofluorescent BrdU double-labeling in conjunction with TuJ1 or NeuN, free-floating sections were denatured in 2 M HCl in 0.1 M TBS for 30 min, rinsed in TBS (pH 7.5), and incubated for 48 h in rat anti-BrdU in TBS (1:200 with 0.5% Tween; Accurate, Westbury, NY) and either mouse anti-TuJ1 (1:500; Covance, Princeton, NJ) or mouse anti-NeuN (1:500; Chemicon, Temecula, CA) at 4°C. Sections were then rinsed thoroughly and incubated in biotinylated anti-rat in TBS (1:250; Chemicon) for 90 min at room temperature. After rinsing, sections were incubated in streptavidin-Alexa 568 in TBS (1:1000; Molecular Probes, Carlsbad, CA) and goat anti-mouse conjugated to Alexa 488 (1:500; Molecular Probes) for 30 min. Finally, sections were rinsed in TBS, mounted onto coated slides, and coverslipped using glycerol in TBS (3:1).
All slides were coded for the duration of the analyses. For peroxidase BrdU analysis in the dentate gyrus, BrdU-labeled cells in the GCL of every 12th section through the unilateral dentate gyrus were counted at ×1,000 using an Olympus (Melville, NY) BX-50 light microscope. For estimates of BrdU-labeled cells per brain, counts were multiplied by 24. To examine the effect of sleep deprivation on another region, the density of BrdU-positive cells was also determined in the caudal SVZ. BrdU cell counts in the caudal SVZ were from three neuroanatomically matched sections through the anterior dentate gyrus per brain. The volume of the analyzed region was determined by using Cavlieri's principle with ImagePro software (Media Cybernetics, Silver Spring, MD) and the data expressed as densities (number of BrdU labeled cells/mm3). For combined BrdU and TuJ1 or NeuN immunofluorescent analyses, 25 BrdU-labeled cells were examined for double-labeling for each marker per brain using a Zeiss Axiovert confocal laser-scanning microscope (lasers argon 458/488 and Hene 543; Zeiss, Oberkochen, Germany) equipped with LSM 510 software.
Statistical Analyses.
Means and SEMs were determined for the above variables (BrdU cell counts, percentages of double-labeled cells, CORT levels, and body weights). For statistical comparison, these values were subjected to either one- or two-way ANOVA followed by Newman–Keuls post hoc analysis.
Acknowledgments
This work was supported by National Institutes of Health Grant MH059740.
Abbreviations
- CORT
corticosterone
- ADX
adrenalectomized
- ADX+CORT
adrenalectomy plus CORT replacement
- CC
cage control
- GCL
granule cell layer
- LP
large platform
- Sham
sham-operated
- SP
small platform
- SVZ
subventricular zone
- REM
rapid-eye-movement sleep
- EEG
electroencephalographic
- TuJ1
class III β-tubulin
- NeuN
neuronal nuclei.
Footnotes
The authors declare no conflict of interest.
References
- 1.Everson CA, Bergmann BM, Rechtschaffen A. Sleep Res. 1988;17:314. [Google Scholar]
- 2.Kushida CA, Everson CA, Suthipinittharm P, Sloan J, Soltani K, Bartnicke B, Bergmann BM, Rechtschaffen A. Sleep. 1989;12:42–46. doi: 10.1093/sleep/12.1.42. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann BM, Everson CA, Kushida CA, Fang VS, Leitch CA, Schoeller DA, Refetoff S, Rechtschaffen A. Sleep. 1989;12:31–41. doi: 10.1093/sleep/12.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Everson CA, Bergmann BM, Rechtschaffen A. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Dallman MF. Brain Res. 1999;851:66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Vining C, Iyer V, Kinni V. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 7.Fadda P, Fratta W. Pharmacol Res. 1997;35:443–446. doi: 10.1006/phrs.1997.0155. [DOI] [PubMed] [Google Scholar]
- 8.Andersen ML, Martins PJF, D'Almeida V, Bignotto M, Tufik S. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 9.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Psychoneuroendocrinology. 2006;31:197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Suchecki D, Tiba PA, Tufik S. J Neuroendocrinol. 2002;14:549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 11.Morden B, Conner R, Mitchell G, Dement W, Levine S. Physiol Behav. 1968;3:425–432. [Google Scholar]
- 12.Romeo RD, Bellani R, McEwen BS. Stress. 2005;8:265–271. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- 13.Armario A, Castellanos JM. J Endocrinol Invest. 1984;7:659–661. doi: 10.1007/BF03349502. [DOI] [PubMed] [Google Scholar]
- 14.Tung A, Takase L, Fornal C, Jacobs B. Neuroscience. 2005;134:721–723. doi: 10.1016/j.neuroscience.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Roman V, Van der Borght K, Leemburg SA, Van der Zee EA, Meerlo P. Brain Res. 2005;1065:53–59. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Guzmán-Marín R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. Eur J Neurosci. 2005;22:2111–2116. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 17.Guzmán-Marín R, Suntsova N, Stewart DR, Gong H, Szymusiak R, McGinty D. J Physiol (London) 2003;549:563–571. doi: 10.1113/jphysiol.2003.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirescu C, Gould E. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 19.Grahnstedt S, Ursin R. Behav Brain Res. 1985;18:233–239. doi: 10.1016/0166-4328(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 20.Cohen HB, Dement WC. Science. 1965;150:1318–1319. doi: 10.1126/science.150.3701.1318. [DOI] [PubMed] [Google Scholar]
- 21.Koban M, Stewart CV. Physiol Behav. 2006;87:1–6. doi: 10.1016/j.physbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Mendelson WB, Bergmann BM. Neurobiol Aging. 2000;21:689–693. doi: 10.1016/s0197-4580(00)00154-8. [DOI] [PubMed] [Google Scholar]
- 23.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Stranahan AM, Khalil D, Gould E. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 26.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 27.Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson HC, Waddell BJ. Endocrinology. 1997;138:3842–3848. [Google Scholar]
- 29.Krieger DT. Endocrinology. 1973;93:1077–1091. doi: 10.1210/endo-93-5-1077. [DOI] [PubMed] [Google Scholar]
- 30.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 31.Kochman LJ, Weber ET, Fornal CA, Jacobs BL. Neurosci Lett. 2006;406:259–269. doi: 10.1016/j.neulet.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 32.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 33.Mirescu C, Peters JD, Gould E. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 34.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 35.Hansson AC, Sommer W, Rimondini R, Andbjer B, Stromberg I, Fuxe K. J Neurosci. 2003;23:6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, Melcangi RC, Riva MA. Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 37.Taishi P, Sanchez C, Wang Y, Fang J, Harding JW, Krueger JM. Am J Physiol. 2001;281:R839–R845. doi: 10.1152/ajpregu.2001.281.3.R839. [DOI] [PubMed] [Google Scholar]
- 38.Sei H, Saitoh D, Yamamoto K, Morita K, Morita Y. Brain Res. 2000;877:387–390. doi: 10.1016/s0006-8993(00)02708-6. [DOI] [PubMed] [Google Scholar]
- 39.Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 40.Leuner B, Gould E, Shors TJ. Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 41.Aimone JB, Wiles J, Gage FH. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 43.Stickgold R. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 44.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 45.Cameron HA, McKay RD. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 45.Cameron HA, Gould E. J Comp Neurol. 1996;369:56–63. doi: 10.1002/(SICI)1096-9861(19960520)369:1<56::AID-CNE4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]