Abstract
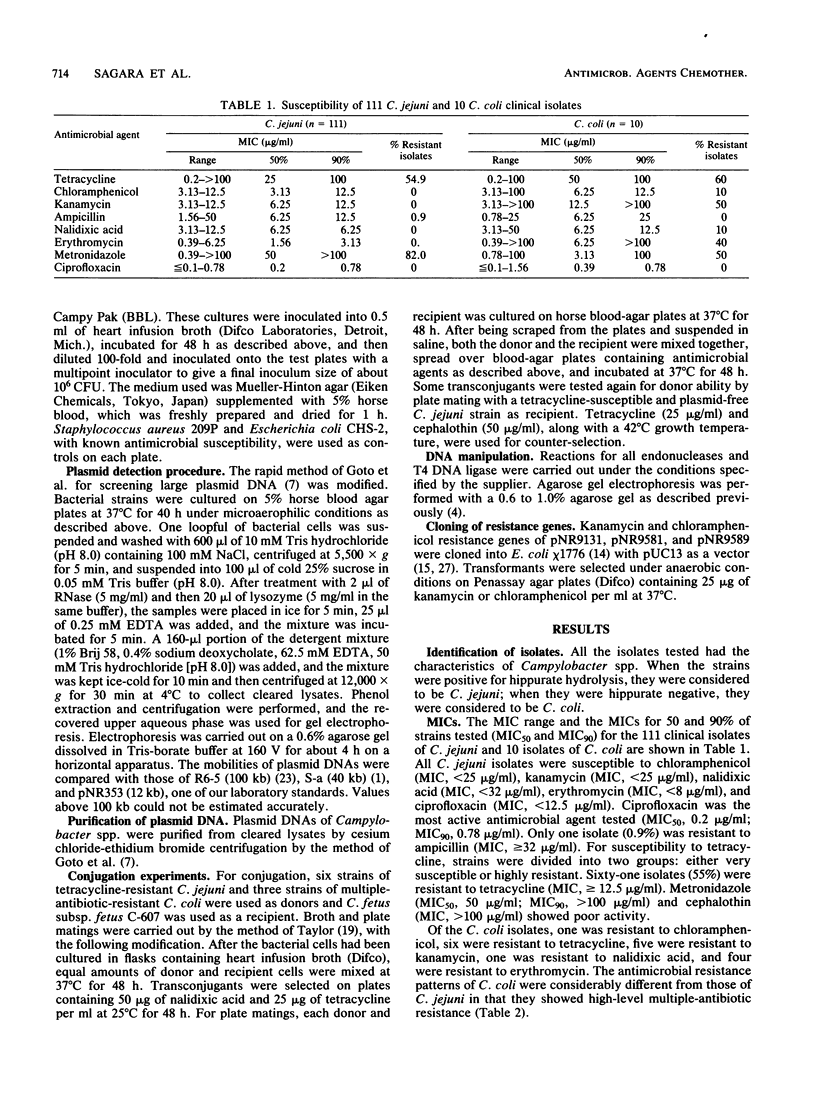
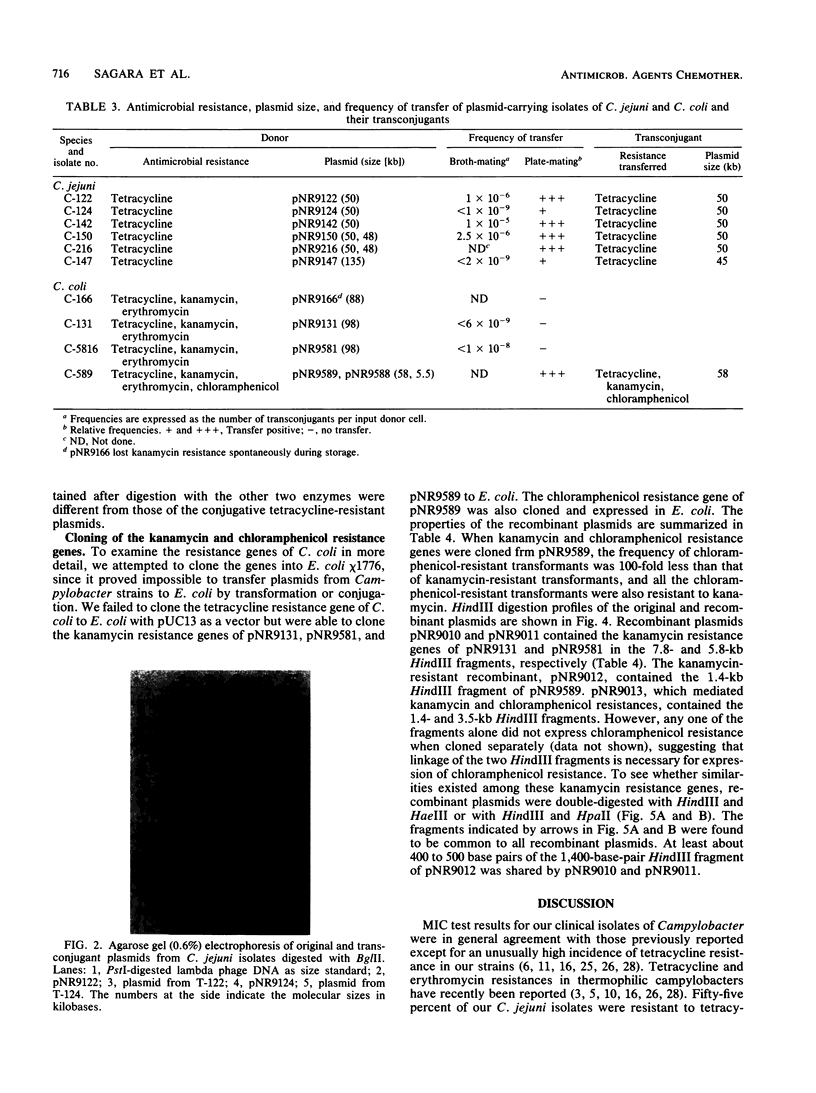
A total of 111 clinical isolates of Campylobacter jejuni and 10 clinical isolates of Campylobacter coli were characterized by their susceptibility to nine antimicrobial agents and by their plasmid profiles on agarose gel electrophoresis. All of the C. jejuni isolates were susceptible to chloramphenicol, ciprofloxacin, erythromycin, kanamycin, and nalidixic acid, but 55% were tetracycline resistant. In the 10 C. coli isolates, a high prevalence of multiple-antibiotic resistance was noted. Plasmids were found in 82% of the tetracycline-resistant and 15% of the tetracycline-susceptible C. jejuni isolates. Tetracycline resistance in six randomly selected C. jejuni isolates, which contained 50- or 135-kilobase (kb) plasmids, was transferred by conjugation to a Campylobacter fetus subsp. fetus recipient with recovery of a 50- or a 45-kb plasmid from transconjugants. From one multiple-antibiotic-resistant C. coli isolate, resistance to tetracycline, kanamycin, and chloramphenicol was transferred concomitantly with a 58-kb plasmid, pNR9589. Nonconjugative 98-kb plasmids, pNR9131 and pNR9581, from C. coli isolates with resistance to tetracycline, kanamycin, and erythromycin were shown by cloning experiments to code for at least kanamycin resistance. Restriction digests revealed that 50-kb plasmids from tetracycline-resistant C. jejuni isolates were identical, although plasmids from multiple-antibiotic-resistant C. coli isolates shared partial DNA homology to each other. Cloning of the kanamycin and chloramphenicol resistance genes of pNR9589 into Escherichia coli showed that the two genes are closely linked or clustered. Double-digestion analysis of the fragments encoding the kanamycin resistance of pNR9131, pNR9581, and pNR9589 showed that these three plasmids contain a common fragment related to kanamycin resistance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Taylor D. N., Feldman R. A. Epidemiology of Campylobacter jejuni infections. Epidemiol Rev. 1983;5:157–176. doi: 10.1093/oxfordjournals.epirev.a036256. [DOI] [PubMed] [Google Scholar]
- Bradbury W. C., Munroe D. L. Occurrence of plasmids and antibiotic resistance among Campylobacter jejuni and Campylobacter coli isolated from healthy and diarrheic animals. J Clin Microbiol. 1985 Sep;22(3):339–346. doi: 10.1128/jcm.22.3.339-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elharrif Z., Mégraud F., Marchand A. M. Susceptibility of Campylobacter jejuni and Campylobacter coli to macrolides and related compounds. Antimicrob Agents Chemother. 1985 Nov;28(5):695–697. doi: 10.1128/aac.28.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegelman R. M., Petrak R. M., Goodman L. J., Segreti J., Trenholme G. M., Kaplan R. L. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob Agents Chemother. 1985 Mar;27(3):429–430. doi: 10.1128/aac.27.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N., Shoji A., Horiuchi S., Nakaya R. Conduction of nonconjugative plasmids by F' lac is not necessarily associated with transposition of the gamma delta sequence. J Bacteriol. 1984 Aug;159(2):590–596. doi: 10.1128/jb.159.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. M. Hippurate hydrolysis by Campylobacter fetus. J Clin Microbiol. 1980 Apr;11(4):435–437. doi: 10.1128/jcm.11.4.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Takahashi M., Kai A., Takano I., Saito K., Ohashi M. [Antimicrobial susceptibility of Campylobacter jejuni and Campylobacter coli isolated from human and animals in Japan]. Kansenshogaku Zasshi. 1984 Nov;58(11):1206–1212. doi: 10.11150/kansenshogakuzasshi1970.58.1206. [DOI] [PubMed] [Google Scholar]
- Karmali M. A., De Grandis S., Fleming P. C. Antimicrobial susceptibility of Campylobacter jejuni with special reference to resistance patterns of Canadian isolates. Antimicrob Agents Chemother. 1981 Apr;19(4):593–597. doi: 10.1128/aac.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Merriwether T. L., Tkalcevic G. T., Gemski P. Genetic studies of kanamycin resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 1986 Aug;30(2):225–230. doi: 10.1128/aac.30.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Michel J., Rogol M., Dickman D. Susceptibility of clinical isolates of Campylobacter jejuni to sixteen antimicrobial agents. Antimicrob Agents Chemother. 1983 May;23(5):796–797. doi: 10.1128/aac.23.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis - the first five years. J Hyg (Lond) 1982 Oct;89(2):175–184. doi: 10.1017/s0022172400070704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with streptococcal class M determinant. J Bacteriol. 1986 Mar;165(3):1037–1039. doi: 10.1128/jb.165.3.1037-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Bronsdon M. A., Gordon K. P., Plorde J. J. Isolation of plasmids encoding tetracycline resistance from Campylobacter jejuni strains isolated from simians. Antimicrob Agents Chemother. 1983 Feb;23(2):320–322. doi: 10.1128/aac.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Vanhoof R., Goossens H., Coignau H., Stas G., Butzler J. P. Susceptibility pattern of Campylobacter jejuni from human and animal origins to different antimicrobial agents. Antimicrob Agents Chemother. 1982 Jun;21(6):990–992. doi: 10.1128/aac.21.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Gordts B., Dierickx R., Coignau H., Butzler J. P. Bacteriostatic and bactericidal activities of 24 antimicrobial agents against Campylobacter fetus subsp. jejuni. Antimicrob Agents Chemother. 1980 Jul;18(1):118–121. doi: 10.1128/aac.18.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Vanderlinden M. P., Dierickx R., Lauwers S., Yourassowsky E., Butzler J. P. Susceptibility of Campylobacter fetus subsp. jejuni to twenty-nine antimicrobial agents. Antimicrob Agents Chemother. 1978 Oct;14(4):553–556. doi: 10.1128/aac.14.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang W. L., Reller L. B., Blaser M. J. Comparison of antimicrobial susceptibility patterns of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Sep;26(3):351–353. doi: 10.1128/aac.26.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]







