DNA bending by proteins appears to be the rule rather than the exception with the topological distortions ranging from tens of degrees to nearly 720° in the nucleosome core (1, 2). Bending is important in DNA packaging and in regulating diverse cellular processes. The Escherichia coli integration host factor (IHF), discovered as a host protein required for lysogeny by bacteriophage λ, plays both of these roles (3, 4). IHF is an excellent model system for analyzing the mechanism by which proteins bend DNA by virtue of its cellular functions, sequence specificity, tight binding, and robust protein-induced DNA bending (Fig. 1 and refs. 5 and 6). The studies of Sugimura and Crothers (7) and Ansari and coworkers (8) in a recent issue of PNAS directly follow the time evolution of DNA bending upon IHF binding to show that bending sequentially follows protein binding.
Fig. 1.
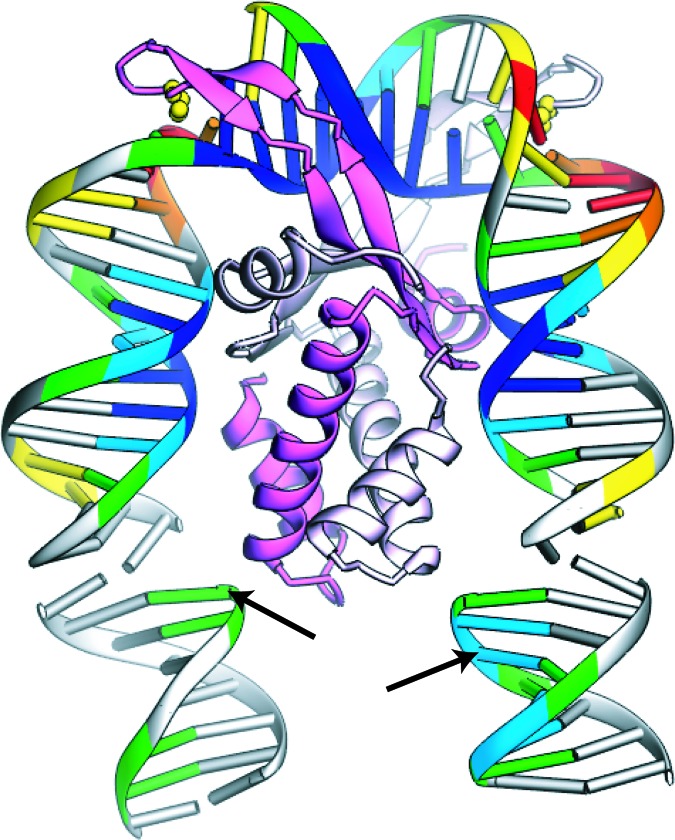
A depiction of the IHF–DNA crystal structure (17) showing the protein subunits in pink (β) and white (α) and the intercalating prolines in yellow. The DNA is color-coded according to the solvent-accessible surface revealed by the hydroxyl radical footprint in solution (5, 14): green, light blue, and dark blue indicate mild, moderate, and strong protection from cleavage, respectively, and yellow, orange, and red indicate mild, moderate, and strong enhancement of cleavage, respectively. Two segments of DNA from symmetry-related complexes within the crystal are included at the bottom to accommodate the full IHF footprint. The attachment positions for the donor and acceptor fluorophores are indicated by arrows.
IHF is a member of a family of Eubacterial proteins that dramatically bend DNA. Although some family members are sequence nonspecific in their binding, IHF binds to specific DNA sequences with nanomolar affinity. DNA structure is important in site selection by all of the family members: DNA bends, nicks, and kinks facilitate binding (6, 10). IHF is a 22-kDa heterodimer of two similar subunits that fold around one another to form a single, compact “body” from which two long β ribbon “arms” extend (Fig. 1). The DNA and protein engage in a mutual embrace: the DNA wraps around the body of the protein whose arms in turn wrap around the minor groove of the DNA.
The IHF-induced DNA bend appears to be stabilized by two mechanisms. First, IHF lines the inside face of the DNA with positive charge, allowing bending by mutual repulsion of the phosphates on the outside face of the DNA. Second, the β ribbon arms reach around to the outside of the complex, where the prolines at their tips intercalate between base pairs, compensating for the disruption in stacking present in the tight bend. The bound DNA entails three relatively straight segments separated by the two large kinks where the proline residues are intercalated.
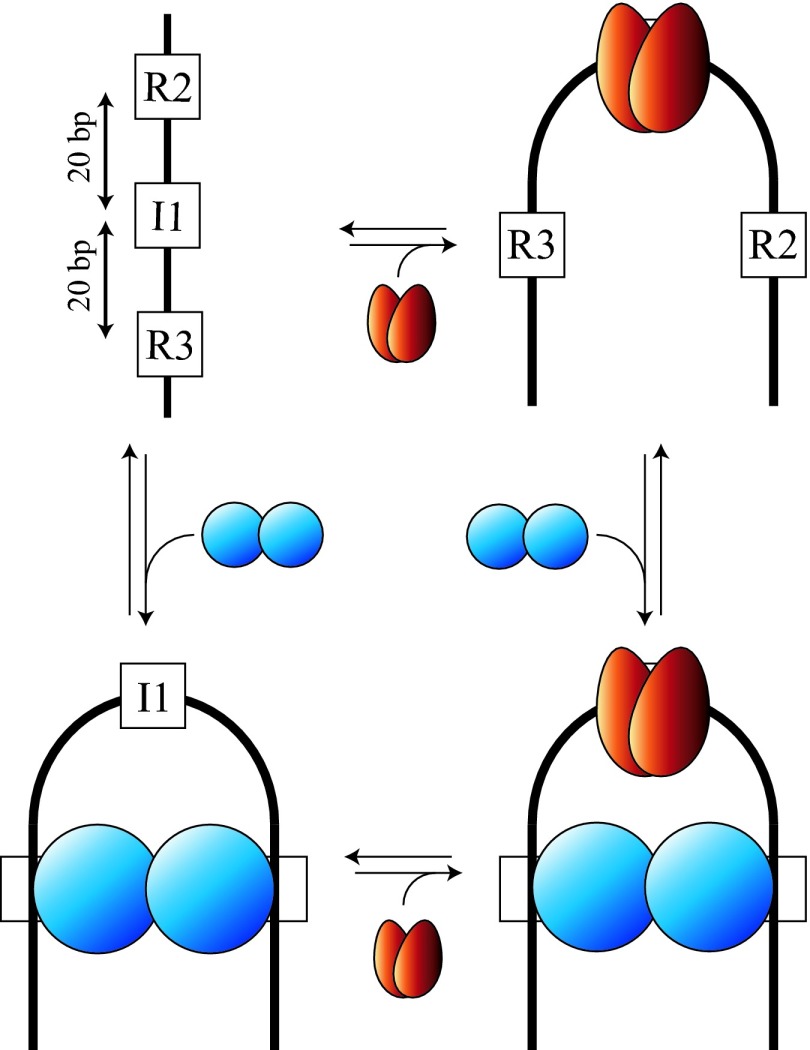
The “U-turn” bend introduced by IHF brings sequences distant along the DNA duplex into spatial proximity (Fig. 1). This geometry consolidates signals in the primary sequence and could facilitate the cooperative binding of regulatory proteins by means of an “indirect” mechanism. Such a mechanism is observed between IHF and the gpNu1 subunit of λ terminase, the viral DNA packaging enzyme. IHF and gpNu1 bind to cos, the packaging initiation site of λ DNA (Fig. 2). IHF-induced bending at the I1 site juxtaposes the two gpNu1 half-sites, facilitating binding. Conversely, binding of a gpNu1 dimer at R3 and R2 introduces a strong bend into the duplex at I1, facilitating IHF binding (Fig. 2 and ref. 9). Cooperative binding of IHF and gpNu1 is thus mediated exclusively by each protein providing a “prebent” architecture without direct interaction. This example illustrates a general mechanism for the assembly of multicomponent nucleoprotein complexes.
Fig. 2.
Model for the cooperative binding and bending of IHF and gpNu1 to their specific sites within cos (Upper Left; I1 and R3/R2, respectively). Bending of the duplex is induced by binding of an IHF heterodimer (red) to I1 and/or by binding of a gpNu1 homodimer (blue) to the R3 and R2 half-sites. Binding of each protein is facilitated by prebending of the duplex. (Adapted from ref. 9.)
Many factors contribute to the stability of IHF–DNA complexes, including relief of conformational strain by the deformability of dinucleotides (12). The free energy of complex formation, including wrapping of the DNA around IHF, drives the DNA conformational change (11–13). Thus, thermodynamic complexity underlies the elegant simplicity of the IHF–DNA complex. Complex reaction kinetics might be expected in light of the global and local structural changes induced in the DNA bound by IHF. Kinetic complexity was observed in time-resolved hydroxyl radical footprinting studies of the association and dissociation of IHF with its specific site on DNA (14). Because hydroxyl radical footprinting monitors the solvent accessibility of the individual nucleotides on the DNA, the conclusion drawn from these studies, that the binding and bending of DNA by IHF is concerted, was inferred from the coincident time-dependent changes in the protection of the three regions of the binding site occluded by IHF (Fig. 1). Limitations of this study were the accessible time resolution and that DNA bending was inferred rather than measured.
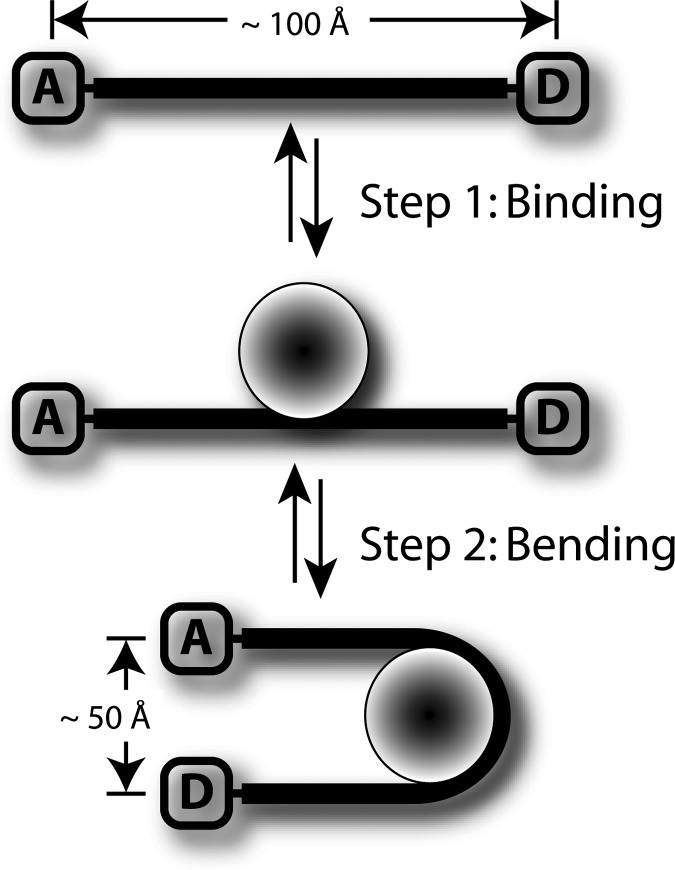
The studies of Sugimura and Crothers (7) and Ansari and coworkers (8) overcome these limitations by directly following DNA bending using FRET with rapid mixing methods sufficient to isolate the unimolecular DNA bending reaction from the bimolecular association of protein with DNA. The sensitivity of FRET comes from the 1/r6 dependence of the fluorescence quenching on the donor–acceptor distance (Fig. 3). Most dye pairs have a critical distance of ≈50 Å and thus can measure distances of ≤100 Å. Another advantage of FRET is the rapid response essential for real-time measurements.
Fig. 3.
A schematic representation of the FRET binding and bending assay. The change in fluorescence as a function of the distance between the donor (D) and acceptor (A) molecules caused by energy transfer (FRET) is the basis of the DNA bending experiments. The distance change designed into these experiments is ideal for the pair of fluorophores used and thus provides a robust measure of DNA bending. This representation does not include the additional DNA present when the substrate molecules were internally labeled. The discrimination of steps 1 and 2 is the seminal result of the studies by Sugimura and Crothers (7) and Ansari and coworkers (8)
In stopped-flow mixing, reactant solutions are rapidly combined and observed within 1 or 2 ms. When IHF was mixed with the labeled DNA duplex bearing its cognate site, the linear increase in reaction rate with protein concentration characteristic of a bimolecular reaction reached a plateau at high IHF concentrations (7). This observation reveals a rate-limiting step in the IHF–DNA association reaction that is presumably submillisecond DNA bending. Further investigation of IHF-induced DNA bending was continued by using a FRET method with microsecond time resolution commensurate with the predicted DNA bending rate. The temperature-jump method uses absorption of near-infrared short laser pulses by water to increase the temperature of medium by ≈10°C within picoseconds to nanoseconds. The perturbed system relaxes to the initial equilibrium at a rate dependent on the enthalpy difference between two states of the system; as much as 10 decades of time are accessible to the temperature-jump method. Because the FRET signal is a direct measure of the distance between the ends of the DNA, the relaxation rate reflects the bending–unbending dynamics of DNA bound to IHF. The rate of DNA relaxation measured by temperature jump is consistent with the rate measured by stopped-flow mixing at the high and limiting IHF concentrations, establishing the consistency of the two approaches (7, 8). The conclusion drawn from these results is clear: formation of the IHF–DNA complex is a sequential two-step process with IHF binding followed by fast DNA bending.
Two sources of the energy necessary to transverse the activation barrier for IHF binding and bending are considered. One source is the local distortions of the DNA structure such as “kinks” and “bubbles,” which can be a consequence of the thermal fluctuations resulting in base-pair openings (7). An alternative hypothesis follows from sequence-specific DNA “bendability” such as that shown for the complex of 434 repressor with DNA (15) and nucleosome assembly (16). The alternative hypotheses are complementary, when the local distortion nucleates sequence-specific DNA bending and the final complex contains DNA either with the kinks as in the IHF–DNA complex (17) or without strongly localized distortion of the structure as in the nucleosome (1).
During the formation of complexes of proteins and DNA, the pathway between the initial and a final states is an arena in which many nonspecific and one specific sequence compete for binding of the protein. Along this pathway, thermodynamically suboptimal but kinetically favorable complexes may be present. The characterization of reaction intermediates can yield fundamental insight into the molecular mechanisms underlying biological processes. Importantly, reaction intermediates provide potential targets for therapeutic intervention. Kinetic approaches exploiting real-time FRET registration illuminate the dynamics of protein–DNA complex formation and DNA bending. The studies of Sugimura and Crothers (7) and Ansari and coworkers (8) describing the kinetics of DNA binding and bending by IHF are a template for the analysis and interpretation of the many proteins that bend the DNA to which they bind to carry out their cellular function.
Footnotes
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Nadassy K, Wodak SJ, Janin J. Biochemistry. 1999;38:1999–2017. doi: 10.1021/bi982362d. [DOI] [PubMed] [Google Scholar]
- 3.Freundlich M, Ramani N, Mathew E, Sirko A, Tsui P. Mol Microbiol. 1992;6:2557–2563. doi: 10.1111/j.1365-2958.1992.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 4.Hendrix RW, Roberts JW, Stahl FW, Weisberg RA. Lambda II. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1983. [Google Scholar]
- 5.Yang CC, Nash HA. Cell. 1989;57:869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]
- 6.Swinger KK, Rice PA. Curr Opin Struct Biol. 2004;14:28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura S, Crothers DM. Proc Natl Acad Sci USA. 2006;103:18510–18514. doi: 10.1073/pnas.0608337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuznetsov SV, Sugimura S, Vivas P, Crothers DM, Ansari A. Proc Natl Acad Sci USA. 2006;103:18515–18520. doi: 10.1073/pnas.0608394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega ME, Catalano CE. Biochemistry. 2006;45:5180–5189. doi: 10.1021/bi052284b. [DOI] [PubMed] [Google Scholar]
- 10.Swinger KK, Rice PA. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.10.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saecker RM, Record MT., Jr Curr Opin Struct Biol. 2002;12:311–319. doi: 10.1016/s0959-440x(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 12.Aeling , Opel ML, Steffen NR, Tretyachenko-Ladokhina V, Hatfield GW, Lathrop RH, Senear DF. J Biol Chem. 2006 doi: 10.1074/jbc.M606363200. 10.1074/jbc.M606363200. [DOI] [PubMed] [Google Scholar]
- 13.Holbrook JA, Tsodikov OV, Saecker RM, Record MT., Jr J Mol Biol. 2001;310:379–401. doi: 10.1006/jmbi.2001.4768. [DOI] [PubMed] [Google Scholar]
- 14.Dhavan GM, Crothers DM, Chance MR, Brenowitz M. J Mol Biol. 2002;315:1027–1037. doi: 10.1006/jmbi.2001.5303. [DOI] [PubMed] [Google Scholar]
- 15.Hogan ME, Austin RH. Nature. 1987;329:263–266. doi: 10.1038/329263a0. [DOI] [PubMed] [Google Scholar]
- 16.Sivolob AV, Khrapunov SN. J Mol Biol. 1995;247:918–931. doi: 10.1006/jmbi.1994.0190. [DOI] [PubMed] [Google Scholar]
- 17.Rice PA, Yang S, Mizuuchi K, Nash HA. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]