Abstract
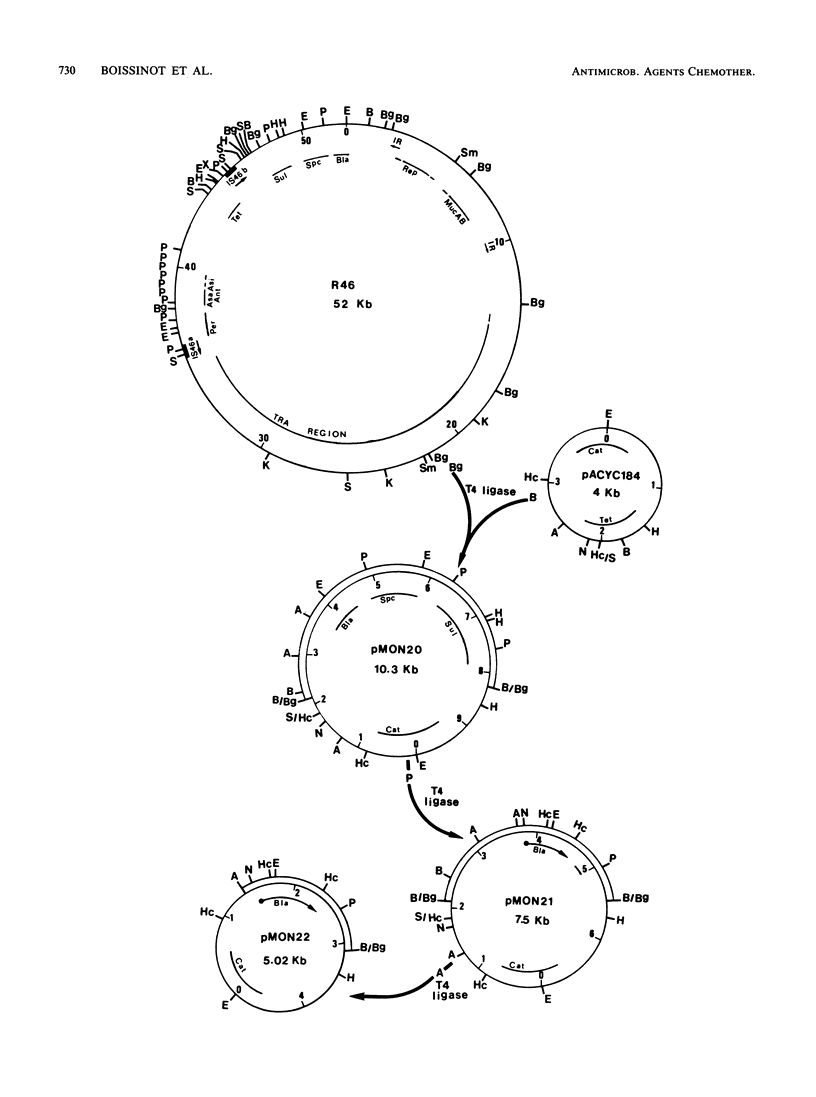
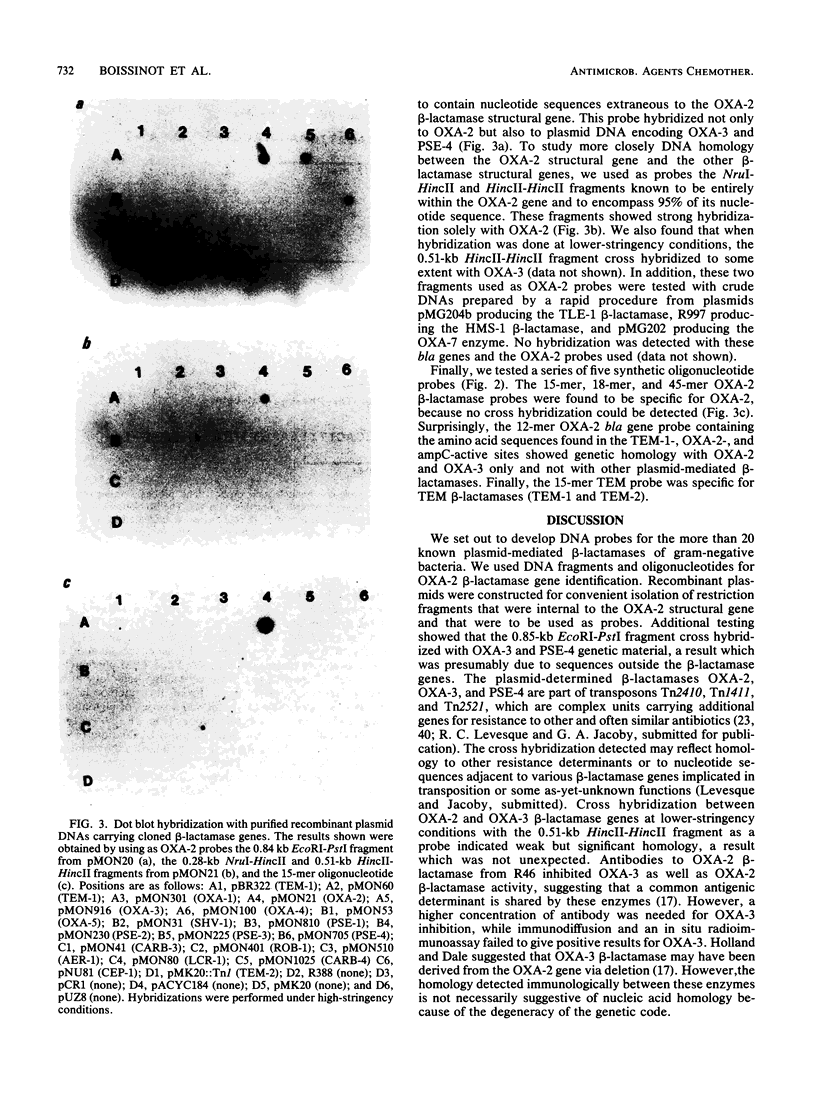
Cloning of a 6.3-kilobase BglII DNA fragment from plasmid R46 permitted the isolation of the OXA-2 beta-lactamase gene. Selected DNA fragments internal and adjacent to the OXA-2 beta-lactamase structural gene were used as probes in homology studies with other plasmid-mediated beta-lactamases. Under conditions of high stringency, no cross hybridization could be detected with DNA probes from within the open reading frame of the OXA-2 structural gene. At a lower stringency, one of two DNA fragments used as probes cross hybridized weakly with the OXA-3 bla gene. Other DNA fragments tested and known to contain sequences flanking the OXA-2 determinant cross hybridized with OXA-3 and PSE-4 plasmid DNA. From the known nucleotide sequence of OXA-2 and TEM-1, we synthesized a series of oligonucleotides corresponding to sequences internal to their respective structural genes. A 12-mer oligonucleotide containing the OXA-2-active-site nucleotide sequences cross hybridized only with OXA-3. All other oligonucleotides tested were found to be specific for their respective OXA-2 or TEM-1 gene. Such beta-lactamase gene probes should facilitate studies of beta-lactamase molecular epidemiology and beta-lactamase gene polymorphism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., DATTA N. RESISTANCE TO PENICILLINS AND ITS TRANSFER IN ENTEROBACTERIACEAE. Lancet. 1965 Feb 20;1(7382):407–409. doi: 10.1016/s0140-6736(65)90004-8. [DOI] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Beltz G. A., Jacobs K. A., Eickbush T. H., Cherbas P. T., Kafatos F. C. Isolation of multigene families and determination of homologies by filter hybridization methods. Methods Enzymol. 1983;100:266–285. doi: 10.1016/0076-6879(83)00061-0. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Coupland G. M., Willetts N. S. Characterization of IS46, an insertion sequence found on two IncN plasmids. J Bacteriol. 1984 Aug;159(2):472–481. doi: 10.1128/jb.159.2.472-481.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Willetts N. S. A physical and genetic map of the IncN plasmid R46. Plasmid. 1981 Mar;5(2):188–201. doi: 10.1016/0147-619x(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B., Mount D. W. Microcomputer programs for DNA sequence analysis. Nucleic Acids Res. 1982 Jan 11;10(1):31–38. doi: 10.1093/nar/10.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Godwin D., Mossakowska D., Stephenson P., Wall S. Sequence of the OXA2 beta-lactamase: comparison with other penicillin-reactive enzymes. FEBS Lett. 1985 Oct 21;191(1):39–44. doi: 10.1016/0014-5793(85)80989-3. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. A direct comparison of two unusual R-factor-mediated -lactamases. Biochem J. 1972 Jun;128(1):173–174. doi: 10.1042/bj1280173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The dimeric nature of an R-factor mediated beta-lactamase. Biochem Biophys Res Commun. 1976 Feb 9;68(3):1000–1005. doi: 10.1016/0006-291x(76)91245-6. [DOI] [PubMed] [Google Scholar]
- Dodd H. M., Bennett P. M. Location of the site-specific recombination system of R46: a function necessary for plasmid maintenance. J Gen Microbiol. 1986 Apr;132(4):1009–1020. doi: 10.1099/00221287-132-4-1009. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Tenover F. C., Young S. A., Gordon K. P., Plorde J. J. Comparison of three DNA hybridization methods for detection of the aminoglycoside 2"-O-adenylyltransferase gene in clinical bacterial isolates. Antimicrob Agents Chemother. 1985 Jul;28(1):69–73. doi: 10.1128/aac.28.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S., Dale J. W. Immunological comparison between OXA-2 beta-lactamase and those mediated by other R plasmids. Antimicrob Agents Chemother. 1985 Jun;27(6):989–991. doi: 10.1128/aac.27.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T., Normark S. Sequence elements determining ampC promoter strength in E. coli. EMBO J. 1982;1(7):875–881. doi: 10.1002/j.1460-2075.1982.tb01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Dideberg O., Charlier P., Wery J. P., Libert M., Moews P. C., Knox J. R., Duez C., Fraipont C., Joris B. On the origin of bacterial resistance to penicillin: comparison of a beta-lactamase and a penicillin target. Science. 1986 Mar 21;231(4744):1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- Kratz J., Schmidt F., Wiedemann B. Characterization of Tn2411 and Tn2410, two transposons derived from R-plasmid R1767 and related to Tn2603 and Tn21. J Bacteriol. 1983 Sep;155(3):1333–1342. doi: 10.1128/jb.155.3.1333-1342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz J., Schmidt F., Wiedemann B. Transposition of a gene encoding OXA-2 beta-lactamase. J Gen Microbiol. 1983 Sep;129(9):2951–2957. doi: 10.1099/00221287-129-9-2951. [DOI] [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Levesque R., Roy P. H. Mapping of the plasmid (pLQ3) from Achromobacter and cloning of its cephalosporinase gene in Escherichia coli. Gene. 1982 Apr;18(1):69–75. doi: 10.1016/0378-1119(82)90057-9. [DOI] [PubMed] [Google Scholar]
- Livermore D. M., Jones C. S. Characterization of NPS-1, a novel plasmid-mediated beta-lactamase, from two Pseudomonas aeruginosa isolates. Antimicrob Agents Chemother. 1986 Jan;29(1):99–103. doi: 10.1128/aac.29.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W., Smith J. T. Types of beta-lactamase determined by plasmids in gram-negative bacteria. J Bacteriol. 1979 Jun;138(3):657–662. doi: 10.1128/jb.138.3.657-662.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., Cohenford M., Jacoby G. A. Five novel plasmid-determined beta-lactamases. Antimicrob Agents Chemother. 1985 May;27(5):715–719. doi: 10.1128/aac.27.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., Levesque R., Jacoby G. A. An animal source for the ROB-1 beta-lactamase of Haemophilus influenzae type b. Antimicrob Agents Chemother. 1986 Feb;29(2):212–215. doi: 10.1128/aac.29.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan C., Holland S., Dale J. W. The interaction of anthraquinone dyes with the plasmid-mediated OXA-2 beta-lactamase. Biochem J. 1982 Aug 1;205(2):413–417. doi: 10.1042/bj2050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Roy P. H. Analysis by using DNA probes of the OXA-1 beta-lactamase gene and its transposon. Antimicrob Agents Chemother. 1986 Jul;30(1):46–51. doi: 10.1128/aac.30.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Bennett P. M., Choi C. L., Brown N., Brunton J., Grinsted J., Wallace L. The genetic basis of the spread of beta-lactamase synthesis among plasmid-carrying bacteria. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):349–359. doi: 10.1098/rstb.1980.0052. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sinclair M. I., Holloway B. W. A chromosomally located transposon in Pseudomonas aeruginosa. J Bacteriol. 1982 Aug;151(2):569–579. doi: 10.1128/jb.151.2.569-579.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid. 1982 May;7(3):239–250. doi: 10.1016/0147-619x(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Young S. A., Tenover F. C., Gootz T. D., Gordon K. P., Plorde J. J. Development of two DNA probes for differentiating the structural genes of subclasses I and II of the aminoglycoside-modifying enzyme 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1985 May;27(5):739–744. doi: 10.1128/aac.27.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]




