Abstract
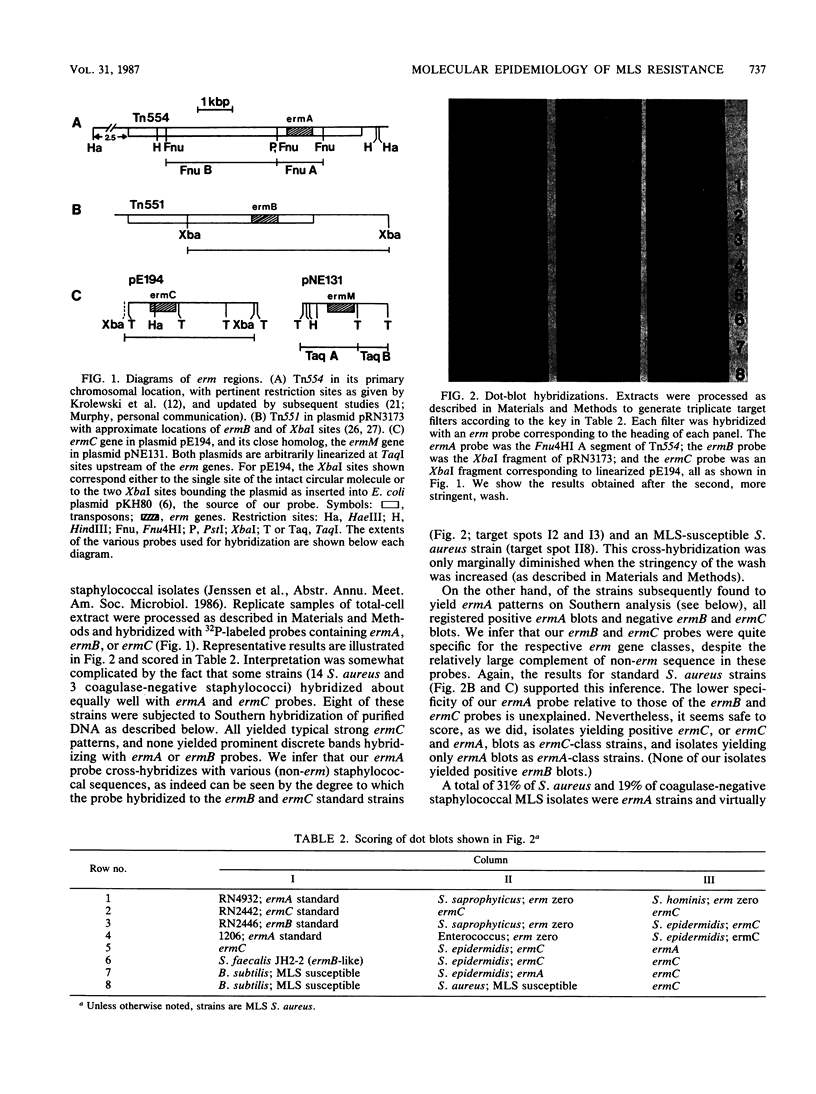
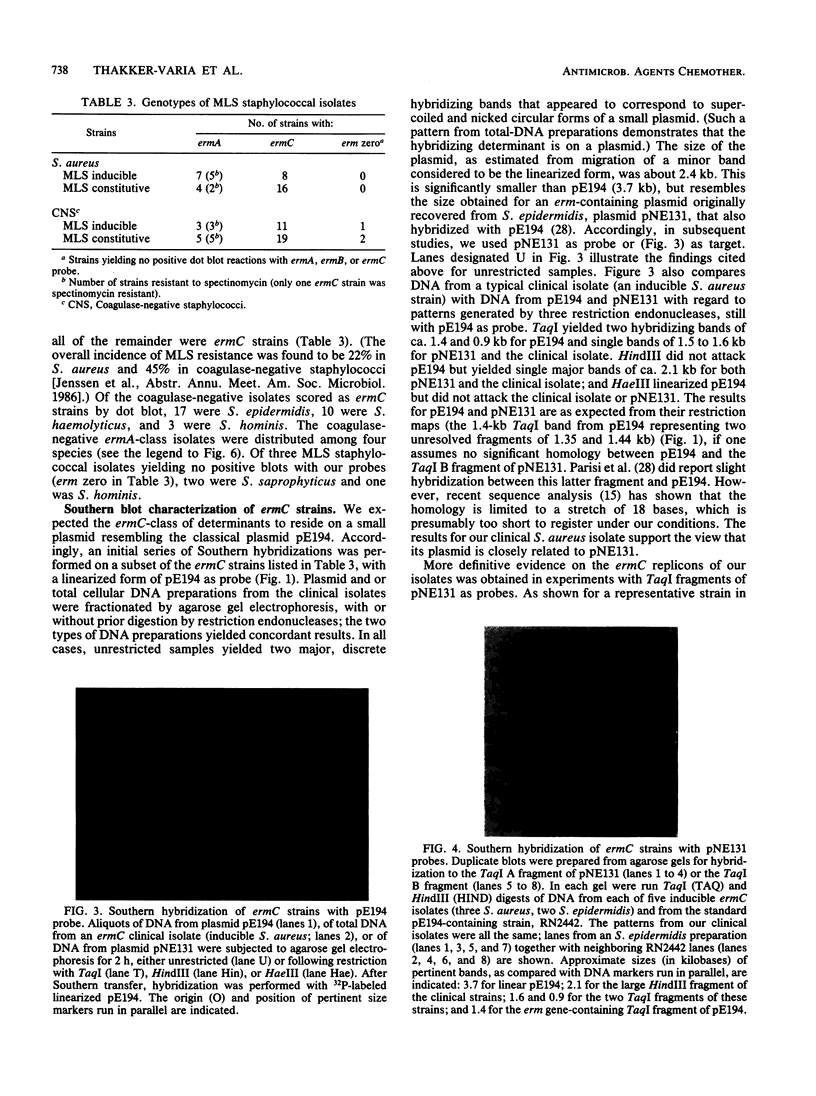
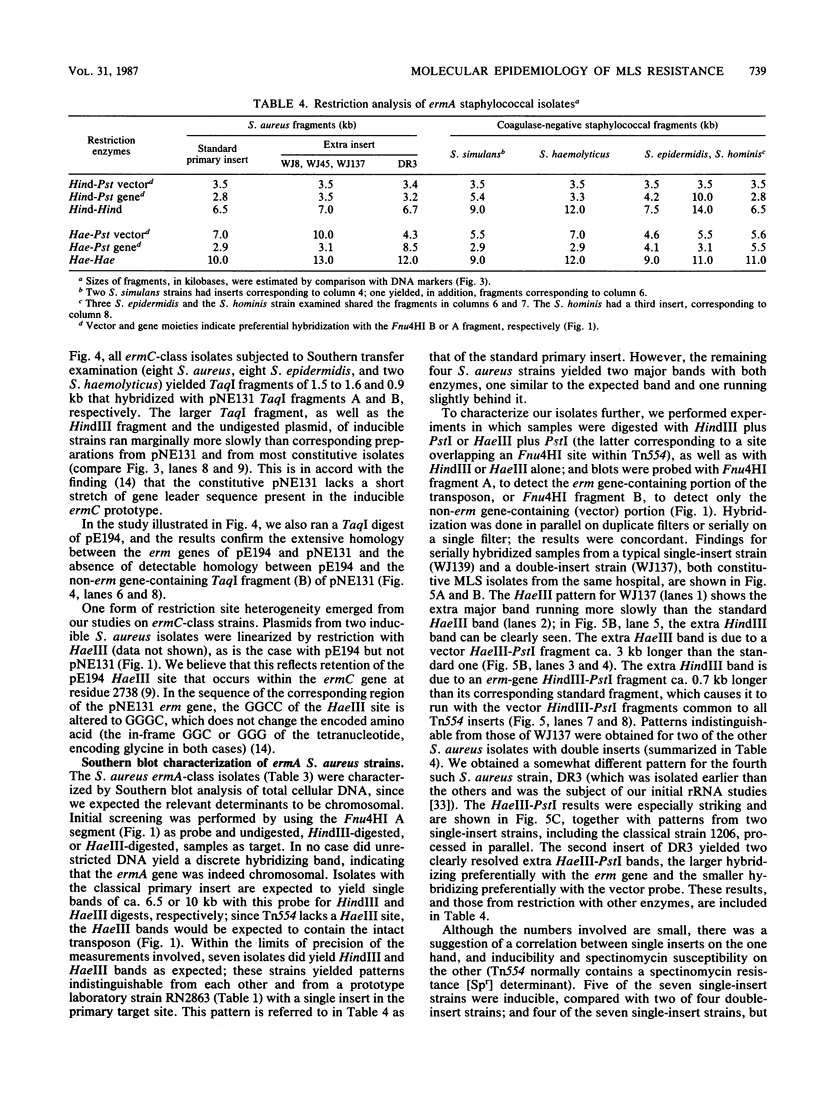
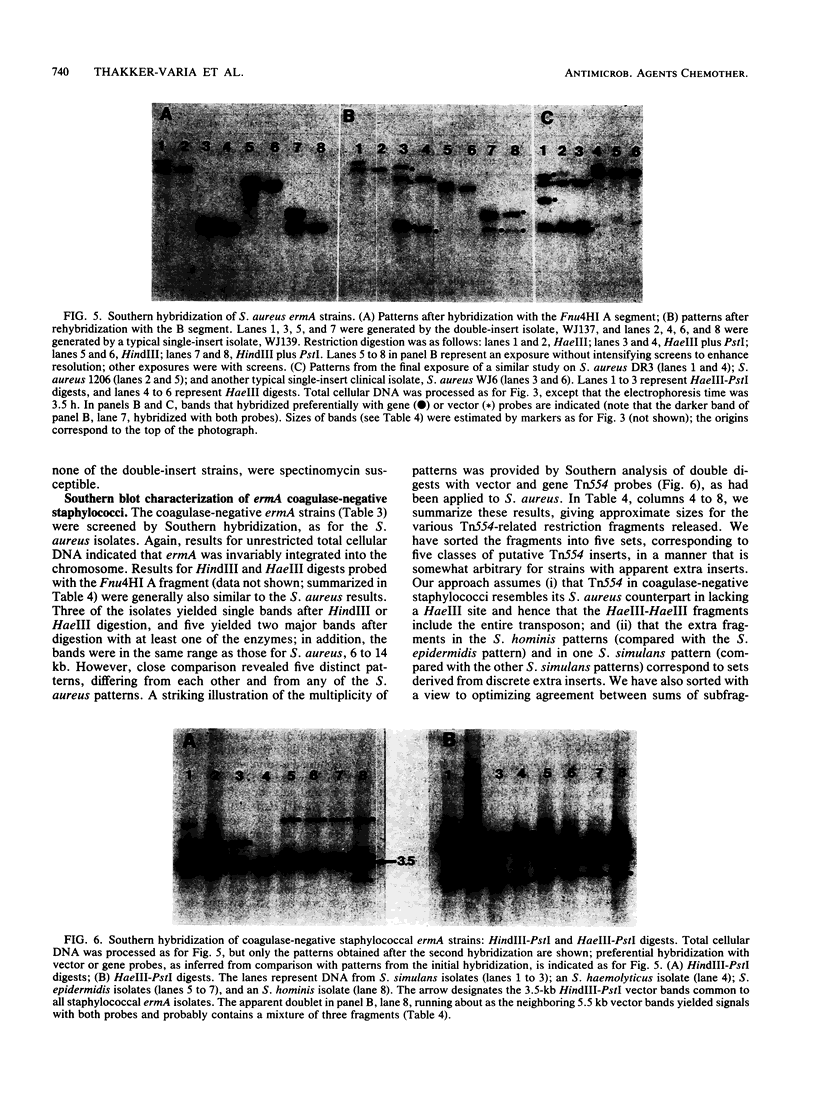
Macrolides-lincosamides-streptogramin B (MLS) resistance is commonly found in Staphylococcus aureus and coagulase-negative staphylococci (22 and 45%, respectively, among isolates from three New Jersey hospitals). We have examined representative subsets of 107 MLS-resistant isolates for the molecular nature of the resistance determinant, the erm gene, by dot blot and Southern hybridization analysis. All of 35 S. aureus isolates examined and 39 of 42 coagulase-negative isolates examined were found to harbor the ermA or ermC evolutionary variant. Genes of the ermC class occurred exclusively on a small plasmid similar to or indistinguishable from one (pNE131) previously described in S. epidermidis. Genes of the ermA class occurred exclusively in the chromosome, and restriction patterns indicated that they were part of a transposon, Tn554, characteristic of the classical S. aureus ermA strain. Unlike S. aureus ermA strains examined previously, which harbor Tn554 at a single specific (primary) site, four of our S. aureus isolates had second inserts at different chromosomal sites. The majority of our coagulase-negative isolates had two or more inserts, neither of which occurred at the classical primary site and many of which differed from one another in location (as inferred from restriction patterns). Coagulase-negative staphylococci constitute a large reservoir of the ermA and ermC class of determinants, with clear potential for interspecies spread.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastos M. C., Bonaldo M. C., Penido E. G. Constitutive erythromycin resistance plasmid in Staphylococcus aureus. J Gen Microbiol. 1980 Dec;121(2):513–516. doi: 10.1099/00221287-121-2-513. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval J. Evolution and epidemiology of MLS resistance. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):137–149. doi: 10.1093/jac/16.suppl_a.137. [DOI] [PubMed] [Google Scholar]
- Hardy K., Haefeli C. Expression in Escherichia coli of a staphylococcal gene for resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1982 Oct;152(1):524–526. doi: 10.1128/jb.152.1.524-526.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Byeon W. H., Weisblum B. A complex attenuator regulates inducible resistance to macrolides, lincosamides, and streptogramin type B antibiotics in Streptococcus sanguis. J Bacteriol. 1983 Jun;154(3):1252–1262. doi: 10.1128/jb.154.3.1252-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Three distinct plasmids originating in the same Staphylococcus aureus strain. Arch Roum Pathol Exp Microbiol. 1976 Jan-Jun;35(1-2):111–118. [PubMed] [Google Scholar]
- Kloos W. E. Natural populations of the genus Staphylococcus. Annu Rev Microbiol. 1980;34:559–592. doi: 10.1146/annurev.mi.34.100180.003015. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Murphy E., Novick R. P., Rush M. G. Site-specificity of the chromosomal insertion of Staphylococcus aureus transposon Tn554. J Mol Biol. 1981 Oct 15;152(1):19–33. doi: 10.1016/0022-2836(81)90093-0. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Antibiotic resistance in Staphylococcus aureus and streptococci. Br Med Bull. 1984 Jan;40(1):77–83. doi: 10.1093/oxfordjournals.bmb.a071951. [DOI] [PubMed] [Google Scholar]
- Lampson B. C., Parisi J. T. Naturally occurring Staphylococcus epidermidis plasmid expressing constitutive macrolide-lincosamide-streptogramin B resistance contains a deleted attenuator. J Bacteriol. 1986 May;166(2):479–483. doi: 10.1128/jb.166.2.479-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson B. C., Parisi J. T. Nucleotide sequence of the constitutive macrolide-lincosamide-streptogramin B resistance plasmid pNE131 from Staphylococcus epidermidis and homologies with Staphylococcus aureus plasmids pE194 and pSN2. J Bacteriol. 1986 Sep;167(3):888–892. doi: 10.1128/jb.167.3.888-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., HASHIMOTO H., KONO M., MORIMURA M. DRUG RESISTANCE OF STAPHYLOCOCCI. II. JOINT ELIMINATION AND JOINT TRANSDUCTION OF THE DETERMINANTS OF PENICILLINASE PRODUCTION AND RESISTANCE TO MACROLIDE ANTIBIOTICS. J Bacteriol. 1965 Apr;89:988–992. doi: 10.1128/jb.89.4.988-992.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Monod M., Denoya C., Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Huwyler L., de Freire Bastos M. do C. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 1985 Dec 1;4(12):3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. Inhibition of Tn554 transposition: deletion analysis. Plasmid. 1983 Nov;10(3):260–269. doi: 10.1016/0147-619x(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Murphy E., Löfdahl S. Transposition of Tn554 does not generate a target duplication. Nature. 1984 Jan 19;307(5948):292–294. doi: 10.1038/307292a0. [DOI] [PubMed] [Google Scholar]
- Murphy E. Nucleotide sequence of ermA, a macrolide-lincosamide-streptogramin B determinant in Staphylococcus aureus. J Bacteriol. 1985 May;162(2):633–640. doi: 10.1128/jb.162.2.633-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Phillips S., Edelman I., Novick R. P. Tn554: isolation and characterization of plasmid insertions. Plasmid. 1981 May;5(3):292–305. doi: 10.1016/0147-619x(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Khan S. A., Murphy E., Iordanescu S., Edelman I., Krolewski J., Rush M. Hitchhiking transposons and other mobile genetic elements and site-specific recombination systems in Staphylococcus aureus. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):67–76. doi: 10.1101/sqb.1981.045.01.013. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E., Gryczan T. J., Baron E., Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979 Jan;2(1):109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Murphy E. MLS-resistance determinants in Staphylococcus aureus and their molecular evolution. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):101–110. doi: 10.1093/jac/16.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Robbins J., Lampson B. C., Hecht D. W. Characterization of a macrolide, lincosamide, and streptogramin resistance plasmid in Staphylococcus epidermidis. J Bacteriol. 1981 Nov;148(2):559–564. doi: 10.1128/jb.148.2.559-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Phillips S., Novick R. P. Tn554--a site-specific repressor-controlled transposon in Staphylococcus aureus. Nature. 1979 Mar 29;278(5703):476–478. doi: 10.1038/278476a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S., Ranzini A. C., Dubin D. T. Ribosomal RNA methylation in Staphylococcus aureus and Escherichia coli: effect of the "MLS" (erythromycin resistance) methylase. Plasmid. 1985 Sep;14(2):152–161. doi: 10.1016/0147-619x(85)90075-7. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N., Labigne-Roussel A., Cohen M. L. Cloned, random chromosomal sequences as probes to identify Salmonella species. J Infect Dis. 1986 Jul;154(1):156–162. doi: 10.1093/infdis/154.1.156. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969 May;98(2):447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]