Abstract
The proinflammatory cytokine TNFα is a potent mediator of septic shock and a therapeutic target for chronic inflammatory pathologies including rheumatoid arthritis and Crohn's disease. As an alternative to anti-human TNFα (hTNFα) mAbs and other hTNFα blocker approved drugs, we developed an active anti-hTNFα immunotherapy, based on a vaccine comprised of a keyhole limpet hemocyanin-hTNFα heterocomplex immunogen (hTNFα kinoid) adjuvanted in incomplete Freund's adjuvant. In mice transgenic for hTNFα (TTg mice), hTNFα kinoid vaccination elicited high titers of Abs that neutralized hTNFα bioactivities but did not result in a cellular response to hTNFα. The vaccine was safe and effective in two experimental models. Kinoid-immunized but not control TTg mice resisted hTNFα-driven shock in one model and were prevented from spontaneous arthritis, inflammatory synovitis, and articular destruction in a second model. These data demonstrate an anti-cytokine induction of autoimmune protection against both acute and chronic hTNFα exposure. They show that active vaccination against a human cytokine can be achieved, and that the immune response can be effective and safe.
Keywords: anticytokine vaccination, rheumatoid arthritis, TNFα therapeutic vaccination, TNFα-dependent shock
The pathogenesis of severe chronic diseases, including rheumatic diseases, autoimmunity, and cachexia, depend at least in part on cytokine dysregulation within altered tissues (1). In particular, the proinflammatory TNFα cytokine was shown to be critically involved in the pathogenesis of rheumatoid arthritis (RA) and further its inhibition by anti-TNFα Abs controls the disease in experimental models (2, 3). These data prompted anti-human TNFα (hTNFα) mAb therapy for RA in humans (4, 5). Efficacy in preventing RA of either mouse–human chimeric (infliximab; ref. 4) or fully human (adalimumab; ref. 5) mAbs was first demonstrated in the transgenic mouse model expressing hTNFα. These mice mimic the clinical and histopathological progression of RA in humans (6, 7). Thereafter, clinical trials using infliximab (8, 9) and adalimumab (10) were successfully completed. Today, these approved therapeutic mAbs, as well as soluble hTNFα receptor derivative (etanercept) specifically targeting hTNFα, are used in therapy of autoimmune diseases (11, 12). About 1 million patients to date have been treated with these TNFα blockers with clinical efficacy in RA (1), Crohn's disease (13), and psoriasis (14). Potential side effects, however, include the risk of infection (sepsis or tuberculosis; ref. 15) and the occurrence of anti-DNA autoAbs (16) and possibly lymphoma (17). Furthermore, these therapeutic approaches are expensive and cumbersome. These limitations prompted investigations of alternative strategies, including active anti-TNFα immunization, targeting TNFα pathogenic effects (18–20). In this report, we show that a biologically inactive but immunogenic hTNFα derivative, constituted of a keyhole limpet hemocyanin (KLH)-hTNFα heterocomplex, called hTNFα kinoid (21), successfully triggers a B but not a T cell response to native hTNFα. Most importantly, this study also proved that induction of polyclonal autoAbs to hTNFα is safe and therapeutically effective in two experimental hTNFα-autologous models. Kinoid immunization inducing anti-TNFα Abs prevents acute TNFα-dependent lethal shock and chronic spontaneous destructive polyarthritis in hTNFα transgenic mice (TTg) mimicking RA.
Results
Biological Properties of hTNFα Kinoid.
Immunobiochemical characterization.
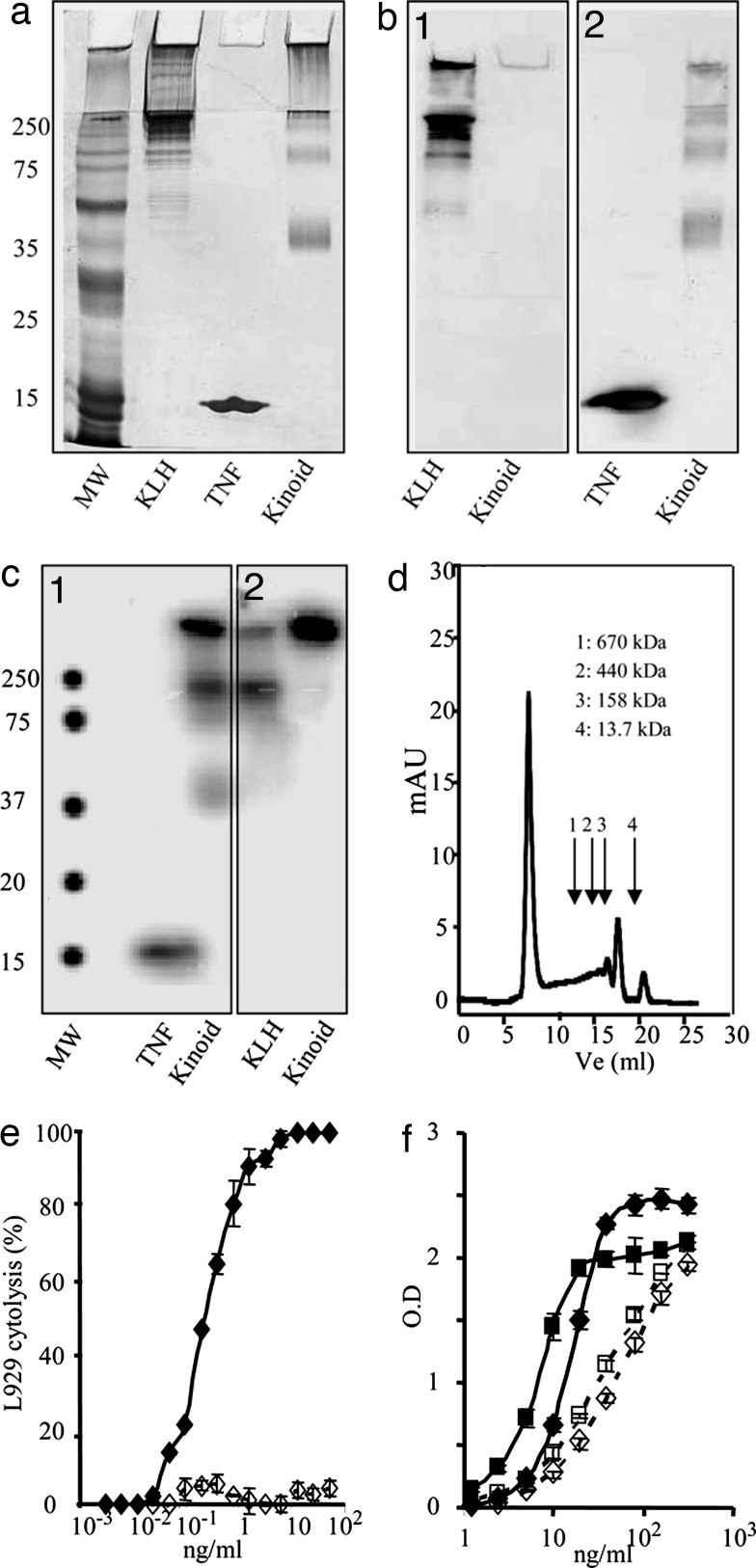
hTNFα kinoid is a KLH–hTNFα heterocomplex that migrated in SDS/PAGE as three bands exhibiting Mr of ≈250, 105, and 40 kDa (Fig. 1a). A rather large proportion of the kinoid did not migrate and is visualized at the level of the stacking gel. Both KLH and hTNFα were identified with specific antibodies (Fig. 1 b1 and b2). A similar diagram of migration was obtained with 125I-labeled kinoid at either hTNFα or KLH, as shown in Fig. 1 c1 and c2. Four peaks were resolved on superose 6 gel filtration. One peak was in the exclusion volume, whereas the three others were of Mr 440, 158, and 13.7 kDa (Fig. 1d).
Fig. 1.
Characterization of hTNFα-kinoid. (a, b1, b2, c1, and c2) SDS (12%)/PAGE. Revelation by silver staining (a); Western blotting with anti-KLH (b1); WB with anti-TNFα (b2); autoradiography of 125-I-TNFα kinoid (c1), and 125-I-KLH-kinoid (c2). (d) Exclusion chromatography on superpose 6 of TNFα kinoid. (e) Comparative evaluation of biological activity of TNFα (♦) and kinoid (◇) on L929 cells. (f) Comparative binding of TNFα to receptor I (♦) and II (■) and of kinoid to receptor I (◇) and II (□).
Absence of TNFα bioactivity.
The kinoid was devoid of any TNFα-induced cytotoxicity, as assessed by standard L929 test even at the highest concentrations (Fig. 1e). hTNFα kinoid bound to both the hTNFα receptor I (p55) and receptor II (p75) despite its desirable lack of bioactivity (Fig. 1f).
Kinoid Induction of Anti-TNFα Abs in TTg Mice.
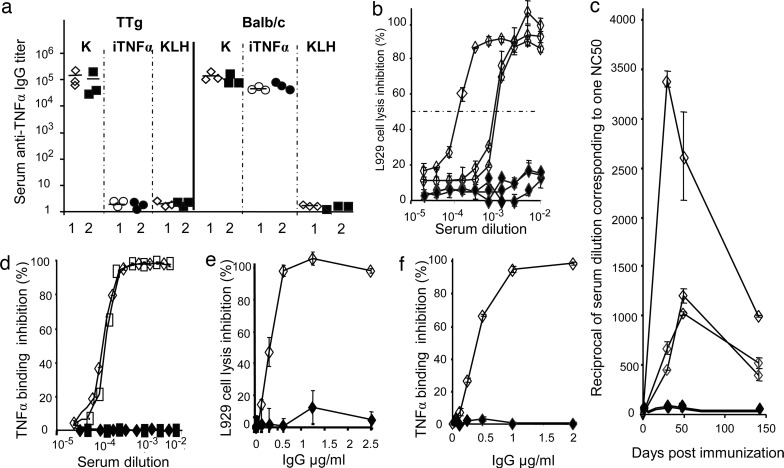
In TTg mice, immunization with TNFα kinoid induced high titers of seric anti-TNFα Abs, whereas immunization with inactivated hTNFα or with control KLH failed to elicit any detectable anti-hTNFα Abs. By contrast, in BALB/c mice, both immunizations with kinoid or with inactivated hTNFα elicited anti-hTNFα Abs (Fig. 2a). In TTg and BALB/c mice, KLH but also kinoid immunizations resulted in the generation of anti-KLH Abs (not shown).
Fig. 2.
Ab response in TTg and BALB/c mice. (a) Serum anti-TNFα Ab titer in kinoid (K), inactivated TNFα (iTNFα), or KLH-immunized TTg compared with BALB/c mice (each point represents the titer in one mouse). Comparison is made between two regimens of immunization: either two i.m. injections of 20 μg 3 weeks apart and boostering (5 μg at 6 weeks) (1) or 2 i.m. injections of 10 μg and a boost of 5 μg (2). (b) Evaluation in kinoid (◇) and KLH (♦) immunized mice of neutralizing capacity according to serum dilution. (c) Serum-neutralizing capacity as a function of time. (d) Serum hTNFα-binding inhibition to receptor I (◇/♦) and II (□/■). (e) IgG-neutralizing capacity evaluated on L929 cells. (f) IgG inhibition of binding to TNFα receptor I. Each point represents an individual serum from a kinoid- (open symbol) or KLH- (closed symbols) treated animal, except in d–f, where pools of three sera provided from kinoid- or KLH-treated mice were used.
As assessed by standard L929 cytotoxicity tests, hyperimmune sera at high dilution from kinoid-immunized TTg mice neutralized TNFα bioactivity even at high dilution, whereas there were no effects of sera from KLH-immunized mice (Fig. 2b). The activity peaked 2–4 weeks postboosting, declined markedly (>50%) within 3 months (Fig. 2c), and blocked the binding of TNFα R1 and R2 receptors (Fig. 2d). It is worth noting that neutralization of mouse TNFα after immunization with hTNFα kinoid could not be achieved. Anti-hTNFα Abs, as tested by ELISA at day 122, belonged mainly to IgG1 (52%) and IgG2a (48%). There were negligible amounts of IgG3, IgM, and IgE. Immune complexes, as looked for by capture ELISA, were not detected in sera of hTNFα kinoid-immunized mice down to 1:10 serum dilution. Finally, purified IgG from hyperimmune sera exhibit high affinity for hTNFα with Kd ranging from 5 × 10−8 M to 10−10 M, as assessed by BIAcore (Uppsala, Sweden) technology, and blocked the binding of hTNFα to its receptor I (Fig. 2f). These results explain why circulating hTNFα was not detected in sera of immunized mice in contrast to its presence in nonimmunized mice at 9 pg/ml, as measured in sera collected 60 days postimmunization.
Absence of Cellular Response to hTNFα.
In vitro culture of splenocytes from kinoid-immunized TTg mice did not trigger any cell-mediated immune response to self hTNFα, as tested by T cell proliferation and cytokine (IL-2 and IFN-γ) production in culture supernatants (Fig. 3). We performed two sets of experiments with TTg mice immunized with hTNFα kinoid or KLH alone on days 0, 7, and 28, and with splenocytes harvested 120 days after priming. We observed the same pattern of results without any in vivo boost (Fig. 3a) or if a boost was performed on day 90 (1 month before death; Fig. 3b). By contrast, kinoid-immunized BALB/c mice exhibited notable cell-mediated immunity to heterologous hTNFα compared with control nonimmunized animals (not shown). No cellular response to mouse TNFα could be detected in immunized mice compared with controls (Fig. 3). hTNFα kinoid immunizations of TTg mice using KLH subunits (provided by Biosyn, Carlsbad, CA) in comparison to KLH (from Intracel, San Diego, CA) confirmed the absence of cellular response to TNFα in both immunizations (not shown). Finally, in immunized TTg mice, a comparable positive T cell response to KLH antigen, equal to that of control animals receiving KLH, was observed that had no detectable clinical effects (Fig. 3).
Fig. 3.
Cell-mediated immunity of mouse splenocytes to hTNFα. Purified splenocytes from KLH- (closed symbols) and kinoid- (open symbols) immunized mice. TTg mice were immunized on days 0, 7, and 28. (a) No boost injection. (b) A boost was performed on day 90. In all mice, spleens were harvested at death (day 122). Splenocytes were stimulated in vitro for 96 h with either hTNFα (h), murine TNFα (mu), or KLH (K). Specific cell proliferation was measured either by 3H-thymidine incorporation and expressed as stimulation index (SI) or IL-2 and IFNγ production in the culture supernatant (in pg/ml) after 44 and 72 h, respectively (see Materials and Methods).
Prevention of TNFα-Galactosamine Lethal Shock by Neutralizing Antibodies Induced by Kinoid Vaccination.
i.p. administration of hTNFα may trigger, in the presence of galactosamine, a lethal shock in mice. The effect is dose-dependent. As shown in Table 1, in C57BL/6 mice, the lethal shock occurred at a dose of 11 μg, and in TTg mice, death was recorded at a dose as low as 1 μg.
Table 1.
Mouse survival at 24 h (alive/total)
| Experiment | hTNFα, μg/mouse | C57Bl/6 |
TTg |
||
|---|---|---|---|---|---|
| Control | Immunized | Control | Immunized | ||
| 1 | 1 | 6/6 | 6/6 | 0/6 | 6/6* |
| 2 | 11 | 0/6 | 6/6 | ND | ND |
| 2 | 2 | 6/6 | 6/6 | ND | 6/6 |
| 2 | 1 | 6/6 | 6/6 | 0/6 | 6/6* |
TNFα-dependent lethal shock: hTNFα in the presence of d-galactosamine was administered by immunoprecipitation in 0.1 ml of PBS 10 days after the last boost to control and kinoid-immunized C57Bl/6 and TTg mice. Prevention of lethal shock as evaluated by animal survival after 24 h was observed only in immunized mice.
∗, P < 0.01 vs. control (χ2). ND, not done.
None of the hTNFα kinoid-immunized C57BL/6 mice treated with 11 μg of hTNFα died, whereas all nonimmunized controls did (Table 1). It is worth noting that the immunized animals not only withstood lethal shock but also remained clinically healthy. Moreover, repeated hTNFα-galactosamine administration at 1-month intervals had no effect (not shown).
Importantly, kinoid-immunized TTg mice also resisted hTNFα-dependent lethal shock, whereas control TTg mice died (Table 1). Administration of higher dosages of hTNFα (2 μg) had no effect on hTNFα kinoid-immunized TTg mice (Table 1). These animals survived after repeated hTNFα shock after 2 weeks and remained fully healthy after 150 days of follow-up.
In immunized mice, protection against shock was due to neutralizing anti-TNFα Abs. Whereas control C57BL/6 mice receiving nonspecific IgG (1 mg) 1 hour after TNFα (11 μg) d-galactosamine administration died within 24 h, mice given specific purified polyclonal IgG from hyperimmune sera survived (P < 0.001 vs. control group). In these experiments, an additional subgroup of control and immunized mice were killed 8 hours post-hTNFα administration, and macroscopic organ analysis showed liver atrophy in control but not in immunized mice (P < 0.02 vs. control; Table 2).
Table 2.
Neutralization of TNFα-dependent shock by hyperimmune IgG
| Mice | hTNFα, μg/mouse | Mouse survival at 24 h, alive/total | Liver atrophy |
|---|---|---|---|
| Control | 11 | 0/10 | 5/5 |
| TNF-kinoid immunized | 11 | 10/10* | 0/5† |
Groups of mice received d-galactosamine and hTNFα by immunoprecipitation in 0.1 ml of PBS 30 min before i.v. injection of protein G-purified mouse IgG from control and kinoid-immunized C57BL/6 mice.
∗, P < 0.001 vs. control (χ2).
†, P < 0.02 (χ2).
TNFα Kinoid Vaccination Protects TTg Mice from Development of Arthritis.
Six-week-old TTg mice were vaccinated with hTNFα kinoid and monitored during a 122-day period. Control groups consisted of KLH- and saline-treated mice, and neither affected the arthritis.
Growth of kinoid immunized mice, as measured by body weight, was comparable to that of control nonimmunized animals up to 50 days postimmunization but became significantly better in later stages (Fig. 4a).
Fig. 4.
Vaccination with hTNFα kinoid improves clinical arthritis in TTg mice. Six-week-old mice were immunized on days 0, 7, and 28 with the KLH–hTNFα heterocomplex (open circles; n = 8) or KLH (closed circles; control group, n = 7). Differences between the groups were statistically significant for any parameter. (a) Evolution of weight gain (gram) from the start of experiment [P < 0.05 (ANOVA)]. (b) Number of affected limbs [Nb of a.l.; P < 0.0001 (ANOVA)]. (c) Clinical scores of arthritis [P < 0.0001 (ANOVA)]. (d) Daily prevalence of arthritis (percent) showing the regression of the disease in vaccinated groups [P < 0.01 (ANOVA)]. This experiment was repeated twice with similar results.
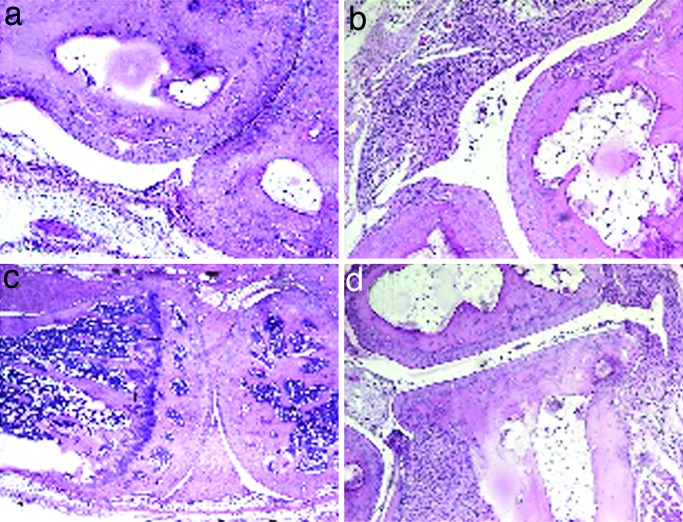
All control mice developed polyarthritis with inflammation and joint deformation (Fig. 4), whereas kinoid-vaccinated mice had only marginal articular disease (P < 0.0001; Fig. 4c), with a delay on onset of clinical signs (P < 0.05), a low score of maximal clinical index (P < 0.01; Table 3), and a grossly less diffuse disease and far less affected limbs (P < 0.0001; Fig. 4b; see also Fig. 6, which is published as supporting information on the PNAS web site). Although all animals developed clinical arthritis, the disease reversed only in the hTNFα kinoid-immunized group (Fig. 4d). At day 120, all animals except one were protected in the treated group (Table 3), in contrast to the control group (P < 0.001). All hTNFα kinoid-treated mice developed high titers of neutralizing antibodies (not shown). Histological evaluation showed a clear reduction of inflammation and joint destruction in immunized animals that exhibited low histological scores (P < 0.01; Table 3); by contrast, control mice showed significant evidence of arthritis, with proliferative synovitis and cell infiltrate with mononuclear and polymorphonuclear cells, associated with cartilage and bone erosion (Fig. 5). The incidences of histological synovitis or destruction were significantly reduced in hTNFα kinoid-vaccinated mice (Table 3; see also Figs. 7 and 8, which are published as supporting information on the PNAS web site).
Table 3.
Clinical and histological scores of arthritis in TNFα kinoid (KLH-TNF) vaccinated and control (KLH) mice
| Immunization | Animal | Clinical evaluation |
Histology |
|||||
|---|---|---|---|---|---|---|---|---|
| Arthritis |
Inflammation |
Destruction |
||||||
| Onset, days | Amax score | Prevalence at day 120 | Score | Prevalence | Score | Prevalence | ||
| KLH | 7 | 36.1 ± 4.5 | 8.6 ± 0.6 | 7 | 1.5 ± 0.1 | 7 | 1.2 ± 0.1 | 7 |
| KLH-TNF | 8 | 49.5 ± 3.2* | 1.4 ± 0.2† | 1‡ | 0.1 ± 0.1† | 0‡ | 0.1 ± 0.1† | 1‡ |
The prevalence of inflammation/destruction as evaluated by histology is the number of mice with a score of inflammation/destruction ≥ 0.5. Amax, maximum arthritis clinical score. Results are given as mean ± SEM.
∗, P < 0.05 vs. KLH.
†, P < 0.01 vs. KLH (Mann–Whitney).
‡, P < 0.001 vs. KLH (χ2).
Fig. 5.
Vaccination with TNFα kinoid improves synovitis and articular destruction in TTg. Examples of histological sections stained with H&E are shown. (a and b) Ankles in vaccinated mice (a; normal aspect) and controls [b; with synovitis (score = 2) and destruction (score = 1.5)]. (c and d) Knees in vaccinated mice (c; normal) and controls [d; with synovitis (score = 1.5) and articular destruction (score = 1.5)]. (Magnification: a and b, ×100; c and d, ×40.)
Discussion
This study shows that preparation of an immunogenic nontoxic hTNFα derivative is feasible and that, after immunization with the kinoid, the induction of anti-TNFα-neutralizing autoAbs safely and effectively controls the pathogenic effects triggered by TNFα in hTNFα-autologous TTg mice. This study has been undertaken within the frame of the development of a novel family of immunogens, namely kinoids, used as the active principle of anticytokine therapeutic vaccines, currently in development to combat cytokine-dependent diseases (22). Kinoid vaccines were developed to trigger B but not T cell responses to abnormally overproduced cytokines, thus to induce high polyclonal Ab titers neutralizing the cytokine and inhibiting its deleterious effects in pathologic tissues (23). The hTNFα kinoid is composed of a KLH–hTNFα heterocomplex chemically prepared by aldehyde treatment, which results in a hTNFα derivative devoid of toxic activities but that is still immunogenic. In the heterocomplex, high-density hTNFα molecules bind to KLH by both covalent and noncovalent linkage (24). We assumed, thereafter, that in heterocomplex units, the KLH partner can supply bystander T cell help by triggering an immune response to foreign KLH antigenic determinants, whereas in this microenvironment, the hTNFα partner with its highly concentrated B cell epitopes triggers crosslinking of B cell receptors. This results in an activation of specific B cells to produce high titers of anti-hTNFα autoAbs, as observed in immunized TTg mice. Interestingly, virus-like particles conjugated to self-TNFα peptides can also overwhelm normal mechanisms for B cell tolerance when present at high density (25).
There were no adverse reactions to the kinoid preparation. The absence of cell-mediated immunity to self-hTNFα was demonstrated in kinoid-immunized TTg mice (Fig. 3). This was expected, given the negative T cell clonal selection to self antigens in the thymus and regulatory T cells controlling self antigens in the periphery and from the absence of autoimmune cell reactions after other anticytokine immunizations, including IL-9 (26), TNFα (3), or other kinoids (22). The rapid decline of anti-hTNFα-neutralizing Abs after kinoid immunization (Fig. 2d) may be due to the short life of B cell memory in the absence of specific T cell help (27).
Another potential safety concern is the occurrence of unimpaired TNFα activity in normal tissues after kinoid immunization. Normal development, a normal growth curve, and a longer survival time together with lack of adverse clinical feature of kinoid-immunized compared with control TTg mice suggest that this is not an issue. The absence of Ab-induced TNFα dysfunction in nontargeted healthy tissues after immunization against self-hTNFα is not surprising. The binding of hTNFα to its receptor at high affinity (Kd = 10−11 M) should not be significantly competed by the limited amount of autoAbs with a lower avidity Kd ≥ 10−10 M present in the thin layer of the slowly circulating lymph in normal tissues. It is also worth noting that the development of TNFα−/− knockout mice is normal despite their greater sensitivity to infection (28).
The therapeutic efficacy of hTNFα kinoid in TTg mice was proven in two experimental models: an acute model of hTNFα-galactosamine lethal shock and a chronic model of inflammatory and destructive arthritis of TTg mice. In the latter, the effect starts early after priming with hTNFα kinoid, as attested by the delay of onset and the separation of weight curves by day 55. Early improvement is fully effective after 60–70 days postpriming, as shown by the reversal of arthritis in hTNFα-vaccinated TTg mice. This long-term observation demonstrates a long-lasting efficiency of an active anti-hTNFα immunotherapy.
In conclusion, these experiments suggest that active immunization against an endogenous cytokine may be a feasible approach in treating pathologies associated with high-TNFα production. They may represent an alternative strategy to passive anti-hTNFα mAb therapy or drugs that block TNFα activity currently used in patients with RA and Crohn's disease. TNFα kinoid vaccination would have the following advantages. First, the generation of polyclonal antibodies by active immunization is unlikely to generate antiidiotypic antibodies, thereby limiting the rate of therapeutic failure (29). Second, the potential low frequency of boost immunization would foster patient compliance. Finally, the proposed therapeutic approach would represent a nonnegligible cost advantage.
Materials and Methods
Reagents.
KLH was purchased from Intracel, except when mentioned; hTNFα was from Boehringer Ingelheim (Mannheim, Germany); murine TNFα was from Cytolab (Rehovot, Israel); hTNFα receptors RI and RII, hTNFα, murine IL-2, and the murine IFNγ ELISA kit were from R&D Systems (Lille, France); and ISA51 adjuvant was from Seppic (Paris, France).
Animals.
Four- to five-week-old female and male heterozygous TTg (1006-T) were purchased from Taconic Farms (Germantown, NY); 6- to 8-week-old female C57BL/6 and BALB/c mice were from Charles River Breeding Laboratory (L'Arbresles, France). Mice were maintained under pathogen-free conditions.
Preparation of Immunogen.
The KLH–hTNFα heterocomplex was prepared as follows. hTNFα (1 mg) in 1 ml of 0.5 M EDTA/0.1 M phosphate buffer, pH 7.8 (working buffer) was treated with 1% DMSO for 30 min. First KLH (0.430 mg) and then glutaraldehyde (0.026 M) were added to this solution. After 45-min incubation at 4°C, the preparation was dialyzed against the working buffer and then treated with formaldehyde (66 mM) for 6 days at 37°C. After quenching with glycine (0.1 M) and subsequent dialysis against Dulbecco's PBS, the preparation was stored at 4°C. The heterocomplex was also prepared with either radiolabeled TNF or KLH proteins using Iodogen (Pierce, Rockford, IL). Native KLH and hTNFα treated as described above were used as control and referenced in the text as KLH and inactivated hTNFα.
SDS/PAGE, Silver Staining, and Immunoblotting.
SDS/PAGE analysis under nonreducing conditions was carried out according to Laemmli (30). On a 12% SDS/PAGE, the proteins were revealed by silver staining, autoradiography (31), or immunoblotting analysis by using the appropriate antibodies.
Gel-Filtration Chromatography.
The heterocomplex was loaded on a Superose 6 10/300 GL gel-filtration column (Amersham Bioscience, Orsay, France) preequilibrated with DPBS and eluted with the same buffer as used for the equilibration, at 0.2 ml/min.
Cell Cultures.
Mouse L929 cell line (American Type Culture Collection, Manassas, VA; CCL 1) was cultured in RPMI medium 1640 containing 10% FCS. Purified murine splenocytes were resuspended in RPMI medium containing 5% FCS and 50 μM 2-mercaptoethanol and incubated in culture medium with or without KLH (30 μg/ml), hTNFα (3 μg/ml), and murine TNFα (1 μg/ml) antigens.
T Cell Proliferation Assay.
Antigen-activated splenocytes were cultured for 4 days and then pulsed for 18 h with 3H-Thymidine. Cells were harvested 18 h later on filter mats, and thymidine incorporation into DNA was quantified by using a β counter. The stimulation index (SI) was expressed as [(mean of cpm from stimulated cells)−(mean of cpm from unstimulated cells)]/(mean of cpm from unstimulated cells). SI values >2 were considered positive.
hTNFα and Receptors Assays.
Direct receptor-binding assay.
The heterocomplex and hTNFα binding to their target receptors were measured by using precoated plates with 50 ng/well of hTNFα RI or RII. Serial dilutions of the samples were incubated with their receptor and bound hTNFα were detected by using biotinylated goat polyclonal anti-hTNFα Ab (R&D Systems). To evaluate the capacity of serum or IgG to inhibit the binding of hTNFα to its receptors, hTNFα was pretreated with serial dilution of tested sera or IgG before being transferred to the plate onto which hTNFα RI and/or RII were immobilized. The blocking titer was expressed as the reciprocal of the serum dilution neutralizing 50% of hTNFα binding.
Anti-hTNFα Ab titer assay.
Specific anti-hTNFα Ab titer in sera of immunized and control mice was determined by a direct ELISA. Precoated ELISA plates with 50 ng/well hTNFα were incubated with serial dilutions of sera from immunized and control mice. Specific IgG were detected by using peroxidase rabbit anti-mouse IgG (Zymed, Carlsbad, CA). Endpoint titers were expressed as the reciprocal of the highest sample dilution giving an OD of 0.3.
Cytokine quantification.
hTNFα, murine IL-2, and murine IFN-γ were determined in the serum and culture supernatant by ELISA.
hTNFα Bioassay.
hTNFα activity was assessed by using the L929 cytotoxicity assay (32). Serial dilutions of hTNFα and heterocomplex were incubated for 18 h with L929 cells in the presence of actinomycin D (1 μg/ml) and the number of surviving cells determined by the MTT dying assay. The ability of hyperimmune serum or IgG to neutralize hTNFα activity was similarly determined after incubating sera and IgG with hTNFα. The neutralizing titer was expressed as the reciprocal of the serum dilution that neutralizes 50% of hTNFα activity.
Immunizations.
Animals were immunized by three or four times i.m. injections of ISA51-adjuvanted kinoid (Seppic) or control preparation (5–30 μg). Sera were collected 8–12 days after the third or fourth immunization and at death.
Antibody Purification.
Ig (IgG) and specific anti-hTNFα or -KLH antibodies were purified from immunized or control mice sera by using a Protein G IgG purification kit (Pierce) or affinity chromatography on hTNFα or KLH-coupled Sepharose 4B columns (Sigma, St. Louis, MO). Avidity was determined by using BIAcore 3000 technology (33).
Lethal Shock.
Control and hTNFα kinoid-immunized mice (C57BL/6 or TTg) were immunoprecipitation-challenged with hTNFα in the presence of 20 mg of d-galactosamine in PBS 10 days after the last immunizing injection. An 80–90% lethal launching dose of hTNFα was administered. Animal survival was recorded 24 h postinjection (34). In these experiments, a macroscopic analysis of the liver was performed in a subgroup of mice killed 8 h post-TNFα administration.
Arthritis Follow-up.
TTg mice 1006-T are similar to Tg197 mice and develop a spontaneous arthritis from the eighth week of age (6). Mice were monitored for evidence of arthritis in four paws by using a blinded procedure. For each limb, clinical severity was scored from 0 (normal) to 3 (severe inflammation with deformation; refs. 2 and 35). The mean arthritis score on each clinical observation day was calculated in each treatment group. For histological analysis, the legs were dissected free and processed as described (36). Extensive sections were cut for each paw, and at least four were examined. The lesions were blindly evaluated for each joint in knee, ankle and foot, as described (37, 38), using a four-point scale (0–3, where 0 is normal and 3 severe) either for synovitis (synovial proliferation or inflammatory cell infiltration) or joint destruction (bone and cartilage thickness and irregularity and presence of erosions).
Statistical Analysis.
All statistics were determined by using StatView version 5.0 software (StatView, Cary, NC). ANOVA was used to analyze repeated measures such as clinical scores, number of affected limbs, weight gain, and prevalence. For comparison of quantitative data, the nonparametric Mann–Whitney test was used. χ2 with Yates correction was used to compare qualitative data.
Supplementary Material
Acknowledgments
We thank Simone Béranger and Monique Etienne (UPRES EA-3410, Université de Paris 13) and Stephane Chambris (animal facilities, Université de Paris 13) for their outstanding technical assistance. This investigation was financially supported by Néovacs S.A. (France) and Debiopharm (Switzerland). We thank Chelatec S.A. (France) for contributions to the performance of the radioactivity assays, BIAcore AB (chiefly to Denis To Van), Néovacs staff (chiefly Patrick Larcier for fruitful discussions), Néovacs technicians for their technical contribution, and Ms. B. Drouet for secretarial assistance. Laure Delavallée is the recipient of a studentship from Association de Recherche sur la Polyarthrite (ARP). This program of ERI18 is funded by Institut National de la Santé et de la Recherche Médicale and Agence Nationale de la Recherche (ANR) and also by Université de Paris 13 and Société Française de Rhumatologie.
Abbreviations
- KLH
keyhole limpet hemocyanin
- RA
rheumatoid arthritis
- hTNFα
human TNFα
- TTg
hTNFα transgenic.
Footnotes
Conflict of interest statement: H.L.B. is a scientist, R.G. a consultant, and D.Z. a shareholder of Neovacs, Incorporated.
This article is a PNAS direct submission.
References
- 1.Feldmann M, Steinman L. Nature. 2005;435:612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 2.Williams RO, Feldmann M, Maini RN. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalum I, Butler DM, Jensen MR, Hindersson P, Steinaa L, Waterston AM, Grell SN, Feldmann M, Elsner HI, Mouritsen S. Nat Biotechnol. 1999;17:666–669. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 4.Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 5.Salfeld JG. Best Pract Res Clin Rheumatol. 2004;18:81–95. doi: 10.1016/j.berh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shealy DJ, Wooley PH, Emmell E, Volk A, Rosenberg A, Treacy G, Wagner CL, Mayton L, Griswold DE, Song XY. Arthritis Res. 2002;4:R7. doi: 10.1186/ar430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, et al. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 9.van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 10.den Broeder A, van de Putte L, Rau R, Schattenkirchner M, Van Riel P, Sander O, Binder C, Fenner H, Bankmann Y, Velagapudi R, et al. J Rheumatol. 2002;29:2288–2298. [PubMed] [Google Scholar]
- 11.Le Loet X, Lequerre T, Vittecoq O. Joint Bone Spine. 2005;72:447–450. doi: 10.1016/j.jbspin.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann M, Maini RN. Nat Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 13.Hommes DW, van Deventer SJ. Curr Opin Gastroenterol. 2003;19:350–357. doi: 10.1097/00001574-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Lancet. 2001;357:1842–1847. doi: 10.1016/s0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 15.Day R. Lancet. 2002;359:540–541. doi: 10.1016/S0140-6736(02)07718-8. [DOI] [PubMed] [Google Scholar]
- 16.Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Arthritis Rheum. 2000;43:2383–2390. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Baecklund E, Askling J, Rosenquist R, Ekbom A, Klareskog L. Curr Opin Rheumatol. 2004;16:254–261. doi: 10.1097/00002281-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Wildbaum G, Youssef S, Karin N. J Immunol. 2000;165:5860–5866. doi: 10.4049/jimmunol.165.10.5860. [DOI] [PubMed] [Google Scholar]
- 19.Capini CJ, Bertin-Maghit SM, Bessis N, Haumont PM, Bernier EM, Muel EG, Laborie MA, Autin L, Paturance S, Chomilier J, et al. Vaccine. 2004;22:3144–3153. doi: 10.1016/j.vaccine.2004.01.064. [DOI] [PubMed] [Google Scholar]
- 20.Chackerian B, Lowy DR, Schiller JT. J Clin Invest. 2001;108:415–423. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bizzini B, Achour A. Cell Mol Biol (Noisy-le-Grand) 1995;41:351–356. [PubMed] [Google Scholar]
- 22.Zagury D, Burny A, Gallo RC. Proc Natl Acad Sci USA. 2001;98:8024–8029. doi: 10.1073/pnas.141224798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zagury D, Le Buanec H, Bizzini B, Burny A, Lewis G, Gallo RC. Cytokine Growth Factor Rev. 2003;14:123–137. doi: 10.1016/s1359-6101(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 24. Neovacs (2004) PCT Patent Application WO 2004/024189. [Google Scholar]
- 25.Chackerian B, Lenz P, Lowy DR, Schiller JT. J Immunol. 2002;169:6120–6126. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 26.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Proc Natl Acad Sci USA. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray D, Skarvall H. Nature. 1988;336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 28.Fantuzzi G. Cytokine Knockouts. 2nd Ed. Totowa, NJ: Humana; 2003. [Google Scholar]
- 29.Anderson PJ. Semin Arthritis Rheum. 2005;34:19–22. doi: 10.1016/j.semarthrit.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Le Roy F, Bisbal C, Silhol M, Martinand C, Lebleu B, Salehzada T. J Biol Chem. 2001;276:48473–48482. doi: 10.1074/jbc.M107482200. [DOI] [PubMed] [Google Scholar]
- 32.Bloquel C, Bessis N, Boissier MC, Scherman D, Bigey P. Hum Gene Ther. 2004;15:189–201. doi: 10.1089/104303404772679995. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson R, Falt A. J Immunol Methods. 1997;200:121–133. doi: 10.1016/s0022-1759(96)00195-0. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann V, Freudenberg MA, Galanos C. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thwin MM, Douni E, Aidinis V, Kollias G, Kodama K, Sato K, Satish RL, Mahendran R, Gopalakrishnakone P. Arthritis Res Ther. 2004;6:R282–294. doi: 10.1186/ar1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bessis N, Guery L, Mantovani A, Vecchi A, Sims JE, Fradelizi D, Boissier MC. Eur J Immunol. 2000;30:867–875. doi: 10.1002/1521-4141(200003)30:3<867::AID-IMMU867>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Saidenberg-Kermanac'h N, Corrado A, Lemeiter D, deVernejoul MC, Boissier MC, Cohen-Solal ME. Bone. 2004;35:1200–1207. doi: 10.1016/j.bone.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Miellot A, Zhu R, Diem S, Boissier MC, Herbelin A, Bessis N. Eur J Immunol. 2005;35:3704–3713. doi: 10.1002/eji.200535235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.